1. Introduction
2. Materials and Methods
Broodstock and gamete production
Cross combination and fertilized egg incubation
Data production and analysis
3. Results and Discussion
1. Introduction
Most groupers (family Serranidae: subfamily Epinephelinae) are important in the aquaculture industry owing to their high economic value. A majority of their global aquaculture production (155K tons/year) is concentrated in China, Taiwan, and Southeast Asia (FAO 2019; Rimmer and Glamuzina 2019). Traditionally, groupers have been sold at high prices in Korean markets, with the kelp grouper (longtooth grouper, Epinephelus bruneus) and red-spotted grouper (E. akaara) particularly sought after for their taste and reddish body color, respectively (Kim et al. 2005). The seeds of these two groupers have been mass-produced for about 10 years, and they are now farmed commercially in sea cages and land-based tanks.
In Korea, however, there are two difficulties related to farming these two groupers. Both species experience retarded growth during low-temperature periods (below 15°C, November to April); accordingly, it takes approximately 3 years for the kelp grouper and red-spotted grouper to reach their minimum marketable sizes (about 1 kg and 0.6 kg, respectively). In addition, even when treated with antibiotics, immunomodulators, chemotherapeutics, and vaccines, almost all individuals of both grouper species die from infections with Vibrio spp. during high-temperature periods in the summer (above 23°C, July to September), when growth rates are high (Harikrishnan et al. 2012).
Interspecific hybrid fish are useful because they can express economically valuable traits through hybrid vigor (Bartley et al. 2000). In particular, the tiger grouper (brown-marbled grouper, E. fuscoguttatus) and giant grouper (E. lanceolatus) hybrid, which inhabits warm water regions, has become commercially successful (Ch’ng and Senoo 2008; Rimmer and Glamuzina 2019). It exhibits higher growth rates and disease resistance than those of the parental species (Bunlipatanon and U-taynapun 2016).
To overcome challenges in grouper farming, fish farmers in Korea have attempted to culture a hybrid between warm water groupers (the tiger grouper ♀ × giant grouper ♂) and two types of hybrids between groupers in temperate and warm water regions (the kelp grouper ♀ × giant grouper ♂ and red-spotted grouper ♀ × giant grouper ♂). However, because of the high mortality of these hybrids during periods of low temperatures, it is only possible to culture them in land-based tank facilities, where hot effluent from power plants (7–9°C warmer than natural seawater) and hot ground water (constant at 16–28°C throughout the year) can be used as heat energy sources.
Therefore, it is necessary to develop the novel grouper hybrid, which has the same viability as the parent species at the temperature in Korea waters, with improved growth and disease resistance through hybrid vigor. As a first step to investigate the possibility of production of the grouper hybrids inhabiting temperate water, the embryonic development and hatchability of fertilized eggs of the reciprocal hybrids of kelp grouper and red-spotted grouper were compared in this study.
2. Materials and Methods
Broodstock and gamete production
Broodstocks of approximately 250 individuals of the kelp grouper and 1,200 individuals of the red-spotted grouper were captured near Jeju and Yeosu and cultured in sea cages for 2–3 years. Females with inflated abdomens and males with milt excretion from their genitalia when their abdomens were pressed were selected, transferred, and stocked in two separate land-based concrete tanks (5 × 5 × 1.5 m). After stocking in land-based tanks, females with oocytes at the vitellogenic stage (oocyte diameter: 300–450 μm) were selected. The oocytes were sampled using biopsy from anesthetized females using catheters (12 Fr; Sewoon Medical Co., Ltd., Seoul, Korea) according to the methods described by Chatakondi et al. (2011) and measured under a microscope (EVOS®XL Core Imaging System, Life Technologies, Carlsbad, CA, USA). For gamete production, a mixture of luteinizing hormone releasing hormone-salmon (LHRH-s, 400 μg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA) and human chorionic gonadotropin (HCG, 500 IU/kg body weight; Daihan Pharmaceutical Co., Seoul, Korea) was injected into the dorsal muscle of females (5–7 individuals of the kelp grouper and 5–10 individuals of the red-spotted grouper) and males (3 individuals of the kelp grouper and 5 individuals of the red-spotted grouper) (Noh et al. 2019). Eggs were collected by hand stripping 36–42 hours after the hormone injection, and milt was obtained within a day of egg stripping and stored at 4°C (Yu et al. 2017). Gamete production was repeated three times during the spawning season in 2015 (trial I in June and trials II and III in July).
Cross combination and fertilized egg incubation
Four crosses were performed in the following combinations, including two hybrids and maternal purebreds in each trial: the kelp grouper ♀ × red-spotted grouper ♂ (KGRG), red-spotted grouper ♀ × kelp grouper ♂ (RGKG), kelp grouper ♀ × ♂ (KG), and red-spotted grouper ♀ × ♂ (RG). In each trial, high-quality eggs from two females were pooled, divided into two Petri dishes, and fertilized with milt from the same and different species using the dry method (Ghittino 1980). After rinsing, the fertilized eggs from the four crosses were divided into 12 five-liter beakers filled with UV-sterilized and filtered natural seawater, with 3,000 to 5,000 fertilized eggs per beaker, using a chemical spoon (three beakers/cross type in each trial). The mean body weights of the females and males used for the experiments were 4.15±1.56 kg (n = 6) and 6.57±1.78 kg (n = 3) for the kelp grouper and 0.71±0.11 kg (n = 6) and 0.92±0.17 kg (n = 3) for the red-spotted grouper.
The experiments were performed in a temperature- controlled three-room incubator (Daihan Scientific Co., Seoul, Korea). Water temperature was measured every hour using temperature loggers (HOBO® TidbiT v2 Water Temperature Data Logger, Onset Computer Co., Bourne, MA, USA), and salinity was measured at the end of the experiment period using the YSI Pro 2030 (YSI Inc., Yellow Springs, OH, USA). During the experiments, the water temperature and salinity were 23.27±0.76°C and 32.37±0.85 psu (trial I), 23.07±0.65°C and 33.17±0.47 psu (trial II), and 23.40±0.30°C and 33.63±0.50 psu (trial III), respectively. Each trial was conducted in triplicate.
Air stones were used to supply weak aeration to the beakers containing fertilized eggs, and about 80% of seawater at the same temperature was exchanged by siphoning at approximately 6-hour intervals to maintain the water quality.
Data production and analysis
Images of each embryonic developmental stage were obtained after observing about 50 fertilized eggs using two microscopes equipped with a digital camera (EVOS®XL Core Imaging System, Life Technologies, Carlsbad, CA, USA; Leica DM 750, Leica Microsystems, Wetzlar, Germany) and classified based on previous studies (Chen et al. 2018; Iwamatsu 2004). The observed fertilized eggs were disposed of after counting to avoid the effects of handling. Images of deformities observed at each stage of embryonic development were obtained; however, the deformity rate was only investigated for newly hatched larvae (about 50 larvae/beaker, triplicates). Deformities were defined as the absence of a straight body or a distinct head distinguished from the yolk (Okomoda et al. 2017).
The eggs that settled to the bottom of the beakers (unfertilized and dead eggs), which were collected by siphoning when exchanging seawater, and hatched larvae and unhatched developing eggs were fixed at the end of the experiments in 10% neutral formalin (Sigma Aldrich, St. Louis, MO, USA) and counted. Hatching rates were calculated according to the following formula: (100 × no. of newly hatched larvae) / (no. of eggs × fertilization rate). Fertilized eggs were considered dead if they did not hatch within 6 hours of the first hatched larva. The time to hatching was estimated as the time required for about 50% of fertilized eggs to hatch.
For statistical analysis, one-way ANOVA followed by Duncan’s multiple range tests or independent t-tests were used (IBM SPSS Statistics Version 21; SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
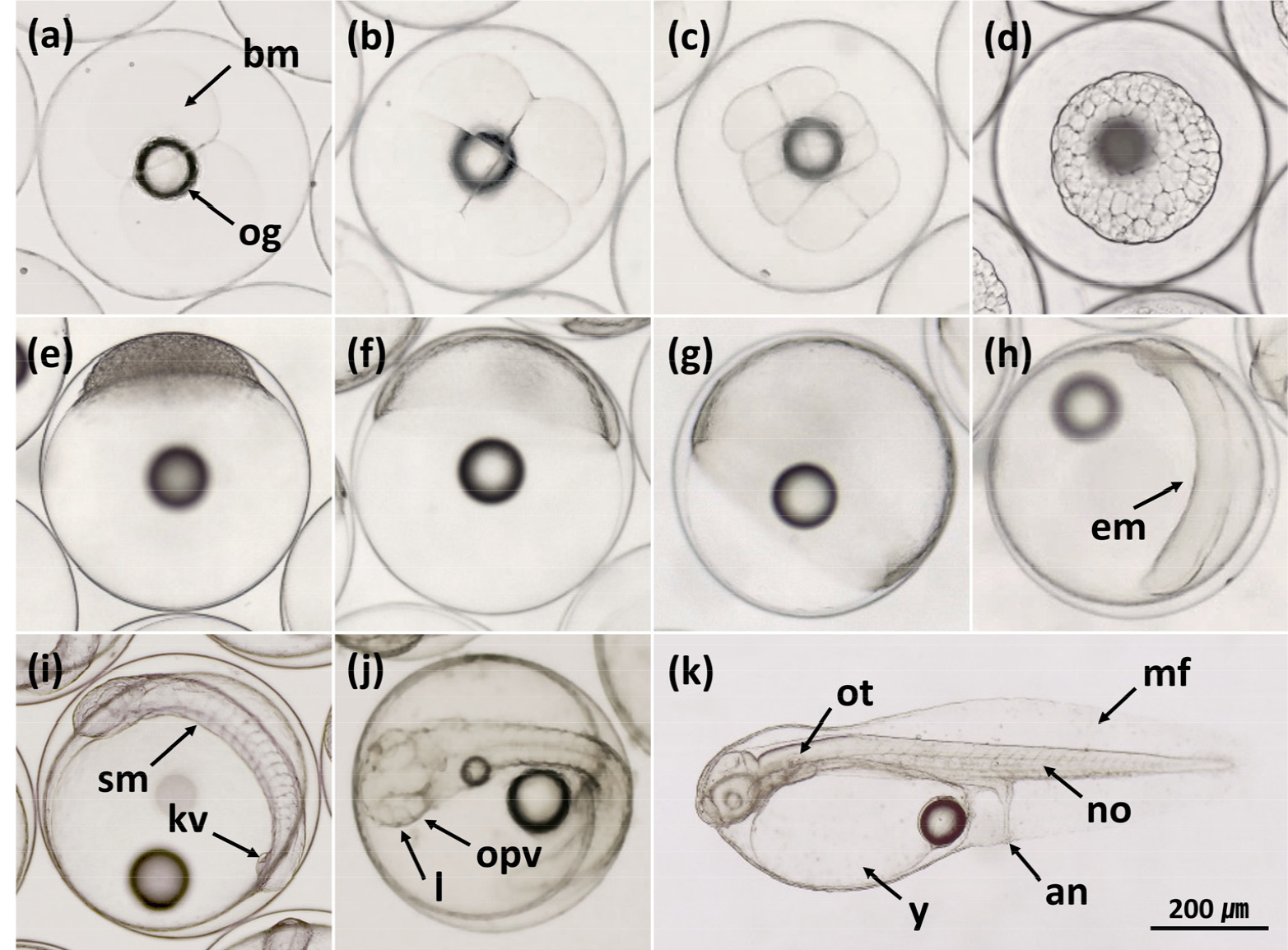
The stripped eggs of the kelp grouper and red-spotted grouper were transparent and spherical. They were separated based on their buoyancy and almost all had a single oil globule. The mean diameters of the stripped eggs were 908.12±21.03 μm (n = 110) for the kelp grouper and 814.41±17.46 μm (n = 130) for the red-spotted grouper.
The fertilization rates of the two maternal purebreds (KG and RG) were not significantly different (p > 0.05, Table 1). However, RGKG had a significantly higher fertilization rate (89.61±1.58%) than that of KGRG (74.82 ±4.23%) (p < 0.05). The hatching rate of fertilized eggs did not differ significantly between the hybrids and maternal purebreds (p > 0.05, Table 1) or between KGRG and RGKG (i.e., 72.74±3.60% and 75.23±2.20%, respectively, p > 0.05). In previous studies, differences in hatching rates between hybrids and maternal purebreds varied among grouper species. The hatching rates of the following hybrids were not different from those of the maternal purebreds: the red-spotted grouper ♀ × giant grouper ♂ (75.56±6.94% vs. 81.54±8.34%, Tian et al. 2015; 76.22± 4.30% vs. 77.17±2.93%, Noh et al. 2019), orange-spotted grouper (E. coioides) ♀ × giant grouper ♂ (51.33±4.91% vs. 48.00±2.89%, Kiriyakit et al. 2011), and goldblotch grouper (E. costae) ♀ × dusky grouper (E. marginatus) ♂ (70% vs. 75%, Glamuzina et al. 2001). On the other hand, the hatching rates of the kelp grouper ♀ × giant grouper ♂ were considerably lower than those of the maternal purebreds (14.35±8.02% vs. 93.60±1.65%, Chen et al. 2018). In this study, the fertilization rates of the two hybrids were lower than those of the maternal purebreds. However, the hatching rates of fertilized eggs of the two hybrids were not lower than those of the maternal purebreds or previously described the grouper hybrids, making these two hybrids desirable crossbreeds.
Table 1.
Parameters of embryonic development and hatchability of the reciprocal hybrids of kelp grouper (Epinephelus bruneus) and red-spotted grouper (E. akaara)
*Uppercase and lowercase letters after values (mean±SD of three trials) indicate the results of an independent t-test (between two crosses with the same female broodstock species) and one‐way ANOVA with Duncan's multiple range test (between all crosses), respectively. Values with the same letter were not significantly different at the 0.05 level.
The fertilized eggs of the two hybrids with normal development showed similar patterns of embryonic development to those of each maternal purebred (Appendix A and B). However, deformities such as irregular cleavage, asymmetric blastoderm, curved spine and shortened body were more frequent in hybrids than in the maternal purebreds (Fig. 1). The deformity rates of newly hatched larvae were 17.47±1.28% for KGRG and 7.11±0.54% for RGKG, which were significantly higher than those of each maternal purebred (p < 0.05, Table 1). In addition, the deformity rate of newly hatched larvae of RGKG was significantly lower than that of KGRG (p < 0.05). In previous studies, the deformity rates of newly hatched larvae of the orange- spotted grouper ♀ × giant grouper ♂ (47.0% vs. 21.33%, Kiriyakit et al. 2011) and kelp grouper ♀ × giant grouper ♂ (85–90% vs. 10–20%, Chen et al. 2018) were higher than those of the maternal purebreds. Deformity rates in the other hybrids were not clearly different from those of maternal purebreds, including the goldblotch grouper ♀ × dusky grouper ♂ (22% vs. 20%, Glamuzina et al. 2001) and red-spotted grouper ♀ × giant grouper ♂ (3.36% vs. 1.58%, Noh et al. 2019).
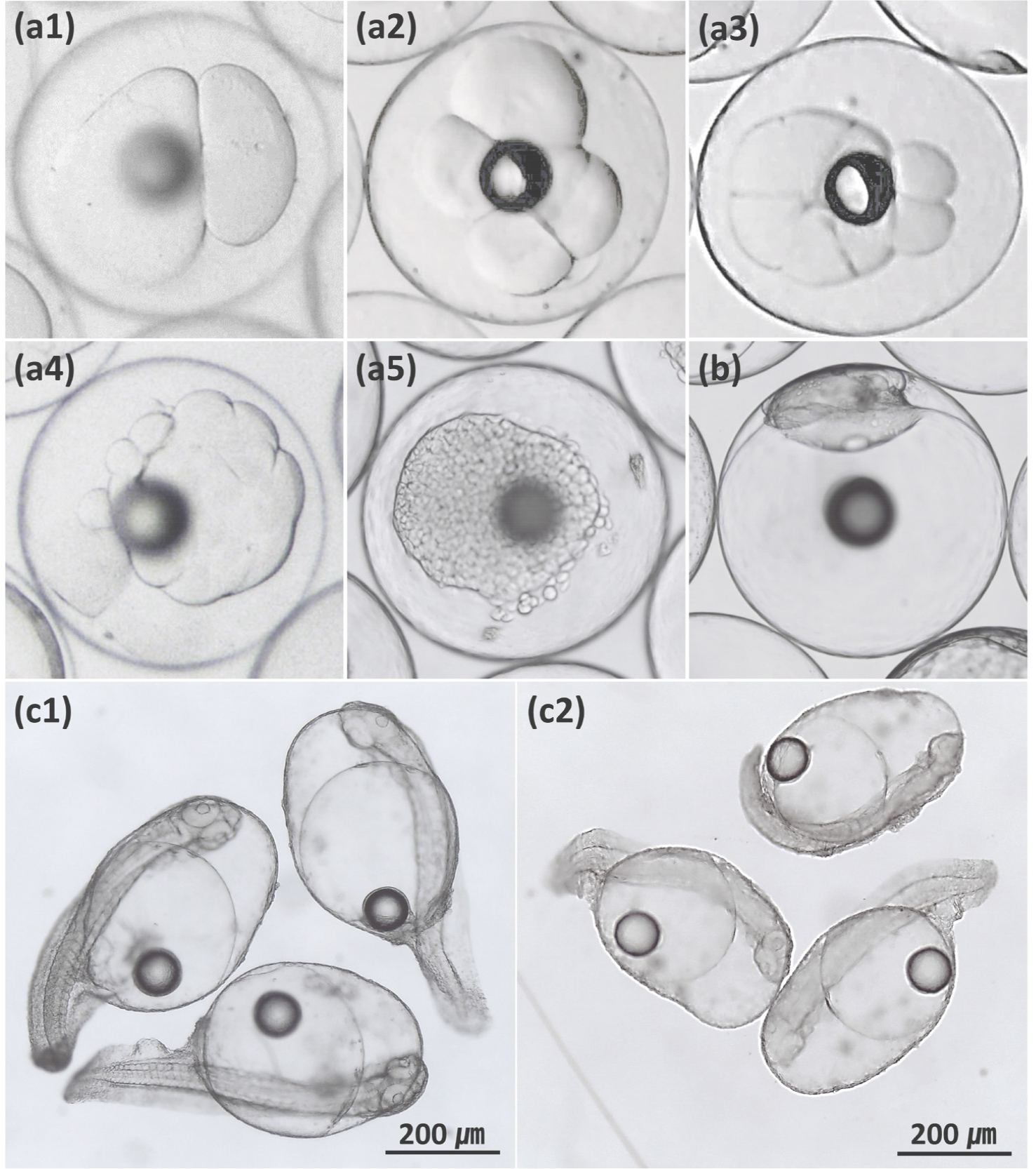
Fig. 1
Deformities of the kelp grouper (Epinephelus bruneus) ♀ × red-spotted grouper (E. akaara) ♂ hybrid. (a) irregular cleavage in 2-cell stage (a1), 4-cell stage (a2), 8-cell stage (a3), 16-cell stage (a4) and morula stage (a5); (b) asymmetric blastoderm; (c) curved spine and shortened body in newly hatched larvae (c1 and c2)
The development of embryonic deformities in fish is determined in part by the quality of gametes, water temperature, and salinity (Bermudes and Ritar 1999; Bonnet et al. 2007; Haddy and Pankhurst 2000). In this study, the water temperature (23.07–23.40°C) and salinity (32.37–33.63 psu) in the embryonic development experiments were within the appropriate ranges (21–25°C and 30–34 psu) for embryonic development in the kelp grouper and red-spotted grouper (Noh et al. 2019; Yang et al. 2007). In the study of Noh et al. (2019), the hatching rate of maternal purebred of the red-spotted grouper was the highest at 25°C to 77.17%, which was no different from that (77.46%) of this study. And Yang et al. (2007) reported that the proper hatching temperature for purebred of the kelp grouper was 24°C. Moreover, maternal purebreds had high fertilization and hatching rates and low deformity rates. Therefore, the high deformity rates of newly hatched KGRG and RGKG larvae can be attributed to specific properties of the species, rather than to egg quality, water temperature, or salinity.
There were no differences in the times to hatching between the hybrids and maternal purebreds, and this parameter was determined by the species of the female. Fertilized eggs from the crosses with the female red- spotted grouper (RG and RGKG) hatched significantly faster than those from the crosses with the female kelp grouper (KG and KGRG) (Table 1, p < 0.05). The time required for fertilized hybrid eggs to develop into fish is the average time for both parental species, similar to one parental species, or, in some cases, slower than the parental species (Chevassus 1983).
In previous studies, the times to hatching for the orange-spotted grouper ♀ × giant grouper ♂, red-spotted grouper ♀ × giant grouper ♂, and kelp grouper ♀ × giant grouper ♂ were not significantly different from the times to hatching for the maternal purebreds (Chen et al. 2018; Kiriyakit et al. 2011; Noh et al. 2019), consistent with the results of the current study. In contrast, the dusky grouper ♀ × white grouper ♂ took hatched 5 hours sooner than the white grouper ♀ × ♂ (Glamuzina et al. 1999).
This study represents a first step toward the development of the novel grouper hybrid. The embryonic development and hatchability of eggs fertilized by reciprocal hybrids of the kelp grouper and red-spotted grouper were analyzed, and the production of viable larvae of the two hybrids was demonstrated. Although normal larvae from striped eggs were obtained more efficiently from RGKG than from KGRG in terms of the fertilization rate, hatching rate, and deformity rate, it is also possible to mass-produce KGRG larvae. Therefore, seed production techniques should be developed for the two hybrids and additional studies of growth performance, disease resistance, deformities during farming and the expression of economically important traits, including body color, are needed to establish the use of these hybrids as alternatives to the kelp grouper and red-spotted grouper in Korean aquaculture.
Appendix
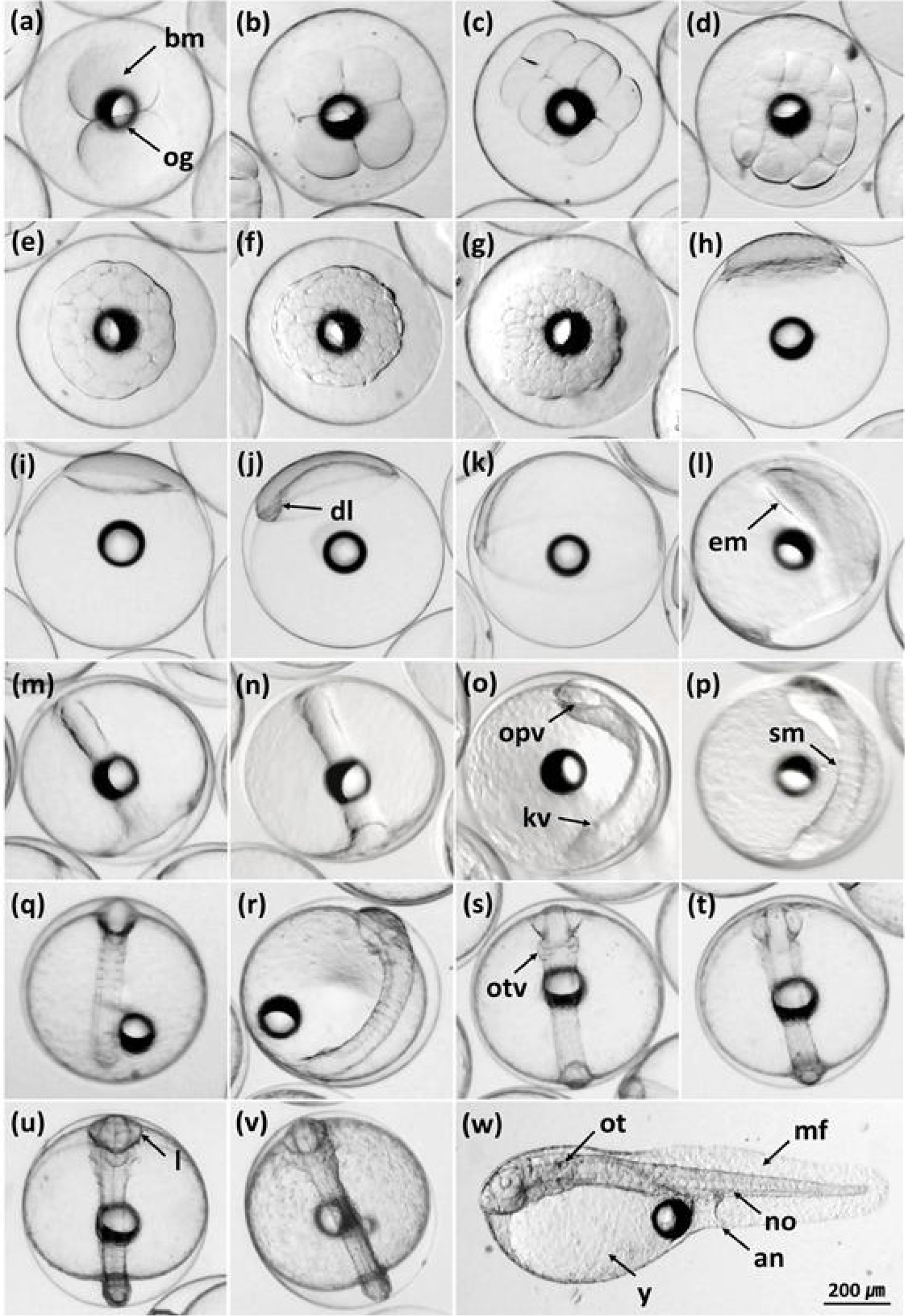
Appendix. A
Embryonic development of the red-spotted grouper (Epinephelus akaara) ♀ × kelp grouper (E. bruneus) ♂ hybrid. (a) 2-cell stage; (b) 4-cell stage; (c) 8-cell stage; (d) 16-cell stage; (e) 32-cell stage; (f) 64-cell stage; (g) morula stage; (h) blastula stage; (i) early gastrula stage; (j) thickened dorsal lip of blastoderm; (k) middle gastrula stage; (l) embryonic body formation; (m) late gastrula stage; (n) completely closed blastopore; (o) appearance of optic vesicles and Kupffer’s vesicle; (p) 5 somites; (q) 6 somites; (r) 7 somites; (s) otic vesicles formation; (t) a groove in each optic vesicle; (u) tail separated from yolk sac and neural fold along the body; (v) tail beating; (w) newly hatched larva. an, anus; bm, blastomeres; dl, dorsal lip of blastopore; em, embryonic body; kv, Kupffer’s vesicle; l, lens; mf, membranous fin; no, notochord; og, oil globule; opv, optic vesicle; ot, otolith; otv, otic vesicle; sm, somites; y, yolk sac