1. Introduction
In the coastal Yellow Sea, the suspension-feeding Manila clam Ruditapes philippinarum occurs at a high density from low intertidal to the shallow subtidal, linking benthic primary production to the upper trophic level. On the west coast of Korea, the well-developed sandy-mud tidal flats are often licensed to the local shellfish unions to explore or culture the clams, where the clam density often reaches over 1,000 individuals/m2 (Park et al. 2013). In particular, Cheonsu Bay and the adjacent coast contain numerous tidal flats, and these areas are utilized as a clam culture ground. South Korea has produced 30,000 to 50,000 tons of Manila clams annually for the past decades, and approximately one-third of the annual landings are known to originate from the tidal flats in the Taean coast and Cheonsu Bay. However, Manila clam landings in Korea have declined for the past twenty years, and recent studies reported various pathogenic organisms associated with Manila clams. Previous studies reported that Manila clams in Korean waters are infected by the protozoan Perkinsus olseni, and the heavy infection retards clam growth and reproduction (Park and Choi 2001; Park et al. 2006; Lee et al. 2020a, 2021).
During summer and early fall in 2004, Manila clams in HD tidal flat in upper Cheonsu Bay exhibited mass mortality. Field survey and laboratory analysis revealed that clams collected from the tidal flats in the late fall were heavily infected by P. olseni and other metazoan parasites (Lee et al. 2021). The poor fitness, such as low condition factor and low carbohydrate levels observed from the clams collected in October 2004, was likely linked to the unusually high level of the parasite infection previously reported from Asia and Europe (Park and Choi 2001; Villalba et al. 2004; Park et al. 2005; Nam et al. 2018; Waki et al. 2018).
We monitored the health condition and pathology of Manila clams distributed in HD tidal flat from April to September 2005, a year after the clam mass mortality occurred, to better understand the impacts of the parasites. This study reports monthly variation in the condition index and histopathology of Manila clams from HD tidal flat.
2. Materials and Methods
Located in the upper Cheonsu Bay (Fig. 1), the study site has a well-developed tidal flat (1.65 × 5.15 km) with numerous tidal channels (Choi et al. 2011). Near the low tide line, Manila clam occurs at a high density reaching 660 clams/m2 (Park et al. 2013). To monitor the health condition of clams, we collected 60 adults clams with shell length (i.e., the longest axis of the shell, SL) 30–40 mm from April to September 2005. After measuring SL, the body tissue was removed and weighed to mg using an electronic balance. Condition Index (CI) was determined as a ratio of the wet tissue weight to the dry shell weight (Uddin et al. 2012; Lee et al. 2020b).
For histology, a 3mm-thick longitudinal section was made in the middle of the body and preserved in Davidson’s fixative. The cross-section included the gills, mantle, foot, and digestive system. After 24 hrs of fixation, the clam tissue went through a series of ascending ethanol to dehydrate then embedded in paraffin. The paraffin blocks were sliced to 5–6 μm, deparaffinized, stained with hematoxylin, counterstained with eosin Y, and examined under a light microscope. Manila clam reproductive phase was categorized into 1) resting, 2) early developing, 3) late developing, 4) ripe, 5) spawning, and 6) spent (Park et al. 2006; Uddin et al. 2012). The percentage of the infected clams was estimated and expressed as infection prevalence.
The infection intensity of P. olseni in Manila clam was determined using Ray’s fluid thioglycollate medium assay (RFTM, Ray 1966). For the assay, a piece of gill tissue was excised from clam and incubated in FTM media according to Park and Choi (2001) and Park et al. (2005, 2006). The FTM incubated clam tissues were dissolved in 2M NaOH, washed several times in phosphate-buffered saline solution (0.15M NaCl, pH = 7.6), and counted P. olseni cell using a hemocyte cell counter (Choi et al. 1989; Yang et al. 2010, 2012). Finally, the infection intensity was recorded as P. olseni cells/g gills.
Monthly means of CI and P. olseni infection intensity were compared using one-way analysis of variance and Duncan’s range test. SAS statistical package was used to run the statistical test. The significant statistical level was set at P < 0.05.
3. Results and Discussions
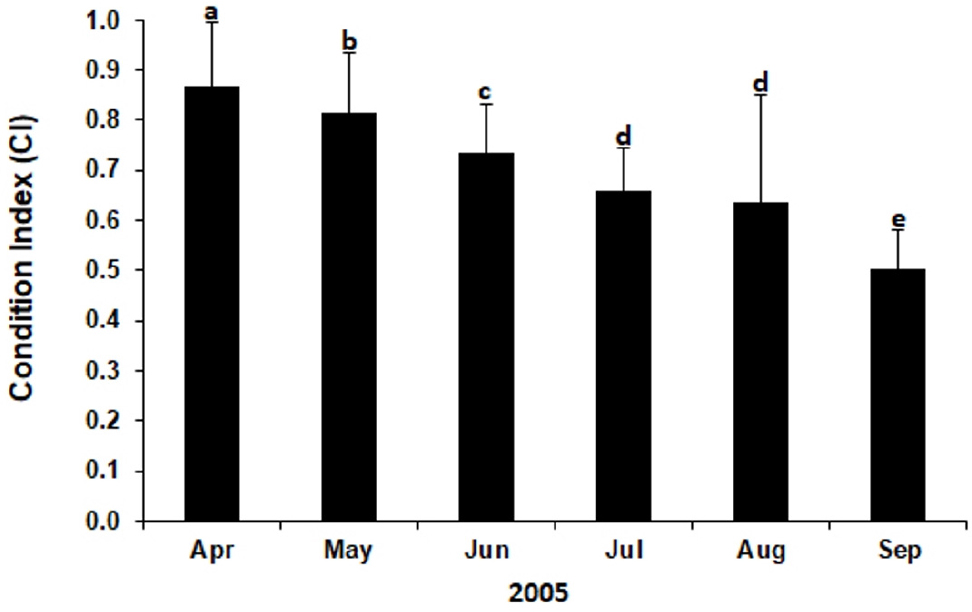
Table 1 summarizes the number and size of clams collected and analyzed in this study. A total of 360 clams with SL ranging from 34.8 to 38.2 mm were collected from April to September 2005. Fig. 1 plots the monthly mean CI of Manila clams in HD tidal flat during the study. CI declined significantly from April (0.87) to June (0.74, P < 0.05). The mean CI determined in September (0.502) was lower significantly than the values determined in other months (P < 0.05).
Table 1.
Summary of the sampling effort
| Year | Month | N | SL (mm)±SD |
| 2005 | APR | 60 | 35.0±2.7 |
| MAY | 60 | 36.7±2.7 | |
| JUN | 60 | 37.5±2.5 | |
| JUL | 60 | 34.8±2.1 | |
| AUG | 60 | 38.2±3.0 | |
| SEP | 60 | 37.3±3.2 |
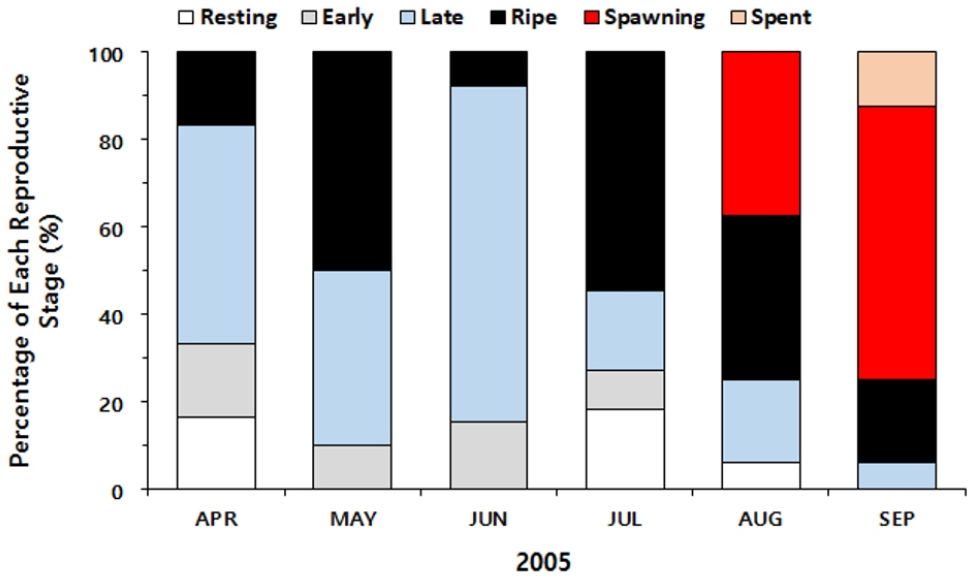
Histology revealed that one-half of the clams in April were in the late developing stage, and 16% were in the ripe stage (Fig. 2). In July, approximately one-half of the clams analyzed were in the ripe stage, and no clams showed signs of spawning. In August, 37.5% of the clams collected were engaged in spawning. Histology also indicated that the spawning continued to September, as 62.5% of the clams were partially spawned (i.e., spawning), and approximately 10% reached the spent stage.
In histology, P. olseni was found dominantly in the gills (Fig. 4). As shown in Fig. 4a and b, numerous clam hemocytes surrounded the P. olseni trophozoites, a typical sign of inflammation, resulting in swollen gill tissues. The trophozoite stage was also observed in the female gonad (Fig. 4c) with a sign of massive hemocyte infiltration. Clusters of P. olseni trophozoites appeared in the connective tissue of the digestive tubules (Fig. 4d), suggesting that P. olseni interferes with the gonad maturation and host food absorption process. The larval trematode of Cercaria tapetis (Park et al. 2008) was observed commonly in the visceral mass and the branchial connective tissues (Fig. 4e). The sporocysts containing developing germinal balls were also common in the visceral mass (Fig. 4f). The metacercaria stage of the trematode Parvatrema duboisi (Cho et al. 2020) occurred at a high density in the mantle epithelial tissue (Fig. 4g). Like P. olseni infection, hemocyte infiltrations were observed along the host tissues occupied by the larval trematodes.
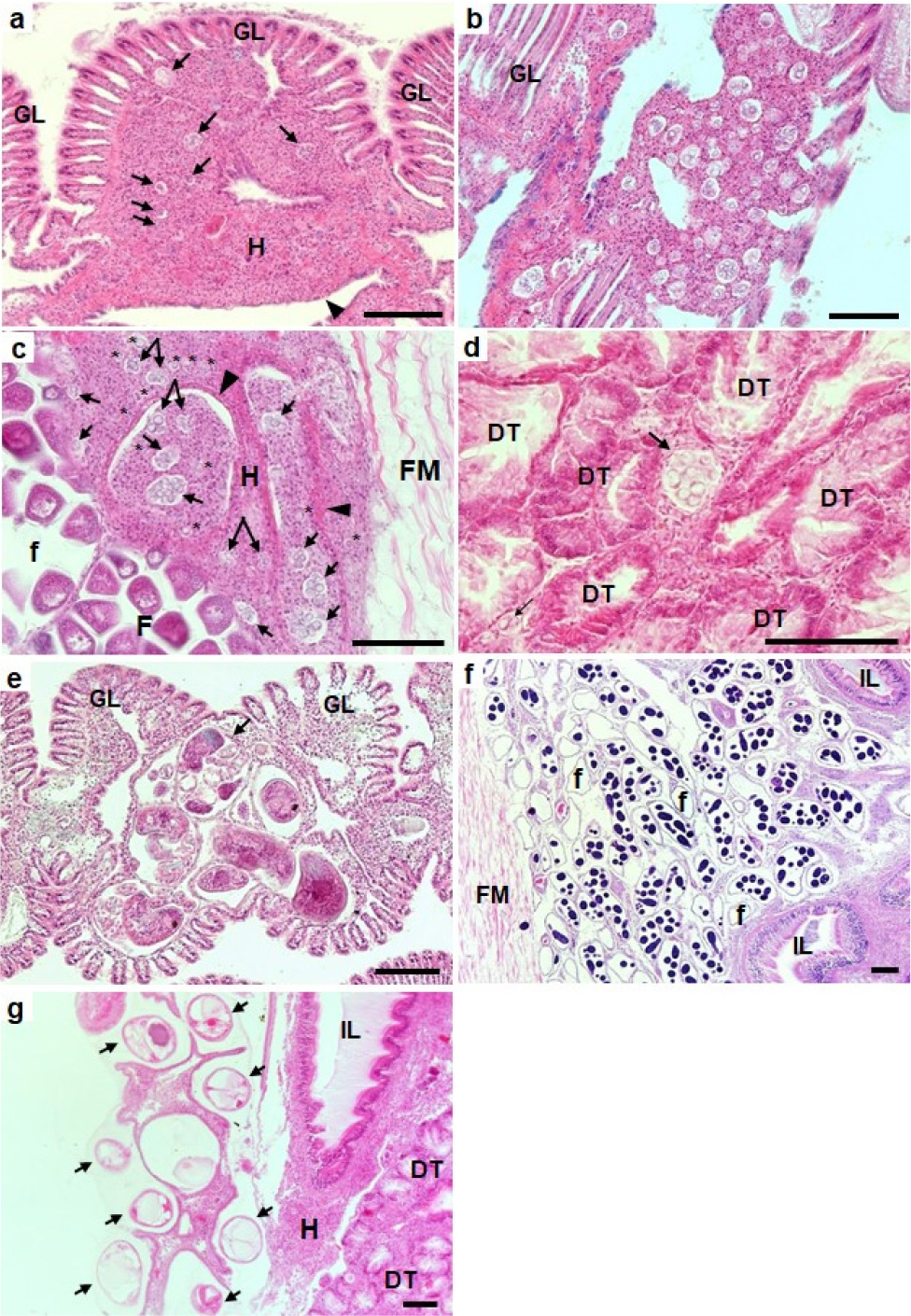
Fig. 4.
Photomicrographs showing Perkinsus olseni (A–D) and trematodes Cercaria tapetis and Parvatrema duboisi. (E–G) appeared in the different host tissues (scale bar = 100 μm). A, clam hemocytes surrounding P. olseni trophozoites (arrows) and heavy infiltration of hemocytes (H and the arrowhead) in the branchial connective tissue of gill lamella (GL). B, numerous P. olseni clusters and hemocyte infiltration in the gills. C, P. olseni trophozoites (* and arrows) in the female follicular connective tissues. D, P. olseni (arrows) in the connective tissues of the digestive tubules (DT), E, sporocysts of mature Cercaria tapetis in the branchial connective tissue of the gill lamella, F, heavy infestation of C. tapetis sporocyst in the gonad, IL, intestinal lumen, G, metacercaria of Parvatreme duboisi in the mantle tissue (arrow)
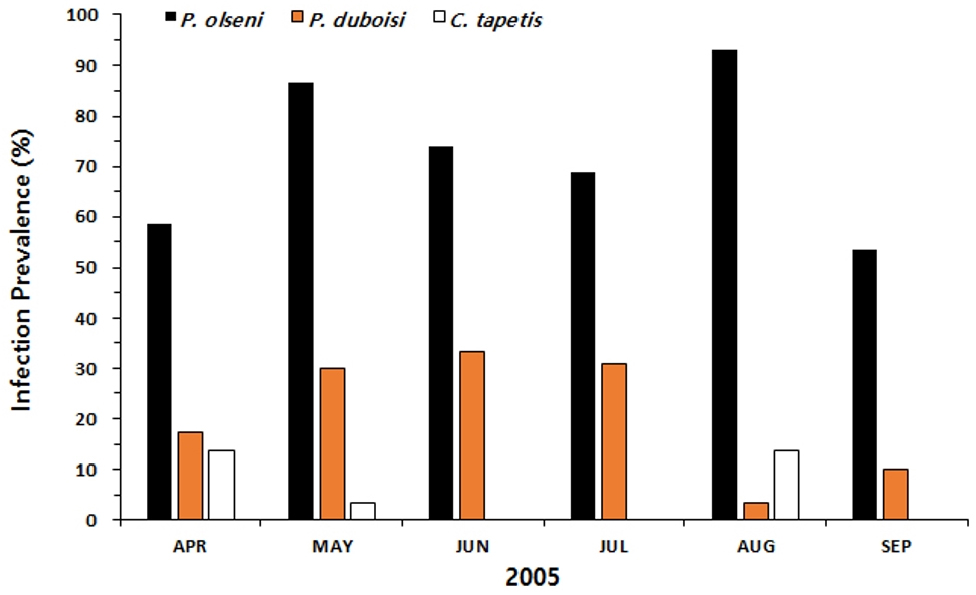
Fig. 5 plots monthly variation in the infection prevalence of P. olseni, C. tapetis, and P. duboisi. The infection prevalence of P. olseni increased markedly from April (58.6%) to May (86.7%) then declined in June and July. The highest P. olseni prevalence was recorded in August at 93.1%. The larval C. tapetis infection prevalence ranged from 3.45 (August) to 33.3% (June), and P. duboisi showed its range from 0 (September) to 13.8% (April and August).
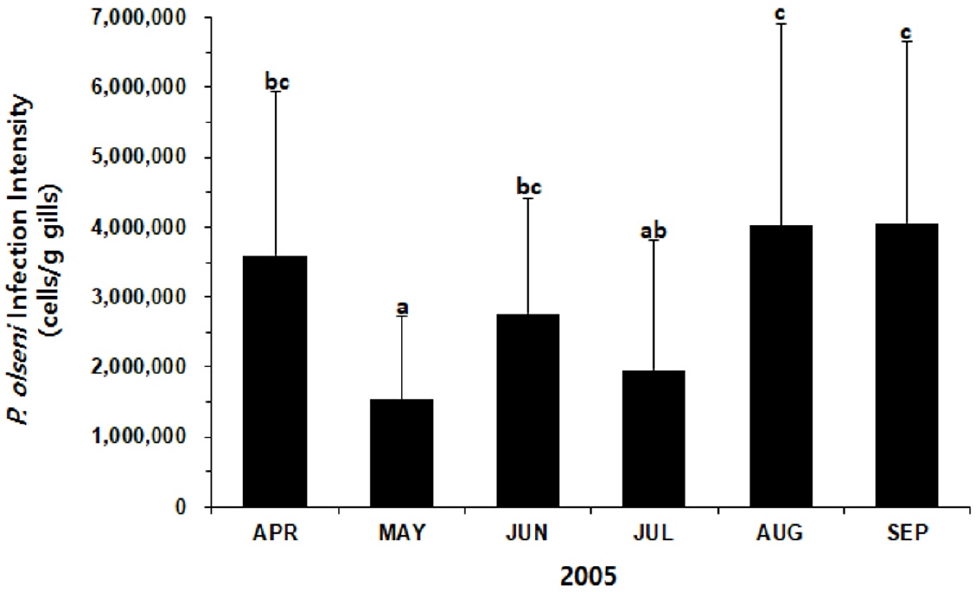
The infection intensity of P. olseni in Manila clams varied from 1.5 × 106 to 4.1 × 106 cells/g gills (Fig. 6). When most clams were actively spawning, the intensity increased dramatically from July (2.0 × 106 cells/g gills) to August (4.0 × 106 cells/g gills). One-way ANOVA and Duncan’s test indicated that the mean infection intensities determined in September and August were significantly higher than the intensities recorded in May and June (P < 0.05).
Lee et al. (2021) first reported parasitic organisms associated with Manila clams in HD tidal flat, where mass mortalities of the clams occurred recurrently for many years. The pathology survey carried out in October 2004 in HD tidal flat, shortly after the mass mortality of Manila clams, revealed that P. olseni heavily infected clams with the prevalence of 100% and the mean infection intensity of 1.74 × 106 cells/g gills. Compared to Lee et al. (2021), the infection intensity measured in August (4.02 × 106 cells/g gills ) and September 2005 (4.07 × 106 cells/g gills is much higher. Park et al. (2006) also monitored P. olseni infection in Manila clams in Gomso Bay, approximately 115km to the south of the current study site. According to Park et al. (2006), P. olseni infection intensity was the highest in October (2.03 × 106cells/g gills), when most clams completed spawning. In addition, the female clams heavily infected by P. olseni in Gomso Bay showed retarded gonad maturation and produced fewer eggs during spawning, suggesting a sublethal effect of a high level of P. olseni parasitism on the host health.
CI of Manila clams in HD tidal flat declined continuously from April to July when the clams were ready to spawn. However, contrary to the present study, Uddin et al. (2012) observed a marked increase in CI in Manila clams distributed in Begmiri tidal flat in Incheon Bay from April to July. In Begmiri tidal flat, CI of Manila clam reached the annual highest in July when the clams were ripe and ready to spawn. A high CI prior to spawning in Manila clam was also observed in Seonjaedo Island tidal flat in Incheon Bay, where Manila clam exhibited the highest CI in May as the clams were fully ripe and ready to spawn (Uddin et al. 2010). The gradual decrease in CI observed in this study can be, in part, explained by the high level of parasitism. As demonstrated by Choi et al. (1989, 1994), the main impact of Perkinsus parasitism on the host is a continuous net energy drain. As a parasite, Perkinsus spp. supply their metabolic energy used in respiration, cell growth, and reproduction entirely from the host. Consequently, host clams or oysters heavily infected by Perkinsus spp. may reserve insufficient net energy for their growth and/or reproduction, resulting in poor fitness (i.e., condition factor or condition index) and slow gonad maturation (Choi et al. 1989, 1994; Lee et al. 2020a).