1. Introduction
2. Material and Methods
Experimental fish
Experimental groups and management
Growth analysis and statistics
3. Results
4. Discussion
1. Introduction
Groupers (family Serranidae, subfamily Epinephelinae) are economically valuable and often commercially reared in Southern China, Taiwan, and Southeast Asia (Rimmer and Glamuzina 2019). Red-spotted groupers (Epinephelus akaara) inhabit tropics/subtropics and temperate regions in Asia (39°N–20°N, 109°E–143°E) (Kaschner et al. 2016); they are valuable because consumers like their reddish body color and taste (Wang et al. 2016). As such, red-spotted groupers have been reared commercially following the establishment of mass-production technologies of fertilized eggs and seeds in an effort to increase aquaculture production in Korea (Lee et al. 2020). However, red-spotted groupers grow slowly. It takes approximately two and three years to reach the marketable size (~500 g) in Jeju, the temperate water region of South Korea and Kaohsiung in Taiwan, respectively (Noh et al. 2019). In order to create a novel seed line that grows fast and has preferred body color, the authors recently induced a hybrid of female red-spotted groupers and male giant groupers and mass- produced juveniles (Noh et al. 2019). Researchers and aquaculturists use hybridization to make hybrid fish with desirable traits of both parental fish (Bartley et al. 2001). Presently, hybridization has been successful between several Epinephelus spp. (Ch’ng and Senoo 2008; Chu et al. 2010; Glamuzina et al. 2001; Kiriyakit et al. 2011); notably, the hybrid between tiger grouper (E. fuscoguttatus) and giant grouper (E. lanceolatus) overcame issues of growth and disease resistance and generated a commercial success (Bunlipatanon and U-Taynapun 2017; Rimmer and Glamuzina 2019).
Water temperature is a critical environmental factor that most influences survival and growth of fish, and is one of first factors to be investigated in order to determine whether a novel aquaculture fish species is industrially useful (Jobling 1997). Obtaining information on optimum temperature is important not only for the rapid growth of a novel breed line but also the exploration of whether a new breed line would be suitable to culture in specific rearing water temperature. The red-spotted grouper prefers water that is 16.0–25.5°C (mean 20.6°C), whereas the giant grouper lives in subtropics (29°N–39°S, 24°E–122°W) and prefers water temperature between 24.3 and 29.1°C (mean 28.1°C) (Kaschner et al. 2016).
This study was undertaken to evaluate the efficacy of novel red-spotted grouper ♀ × giant grouper ♂ hybrid in the aquaculture industry. The effect of hybridization on the optimum temperature to maximize growth performance was investigated by comparing it with the maternal purebred at four different water temperatures.
2. Material and Methods
Experimental fish
Juvenile red-spotted grouper ♀ × giant grouper ♂ (RGGG) and red-spotted grouper ♀ × ♂ (RG) created on July 15, 2015 by fertilizing stripped red-spotted grouper eggs using cryopreserved giant grouper sperm and fresh red-spotted grouper sperm, respectively. The juvenile RGGG and RG were mass-produced in the commercial hatchery of Cheong Sol Hatchery Co. Ltd. in Muan, Jeollanam-do. The juveniles were divided into four different 1-ton cylindrical FRP tanks (diameter 75 cm and height 75 cm) in the indoor aquaculture facility of the East Sea Research Institute, Korea Institute of Ocean Science & Technology, and acclimated to themselves to the new conditions for two weeks before the experiments. During that time, each tank was supplied with recirculated seawater and water temperature was maintained at ~19°C (salinity, ~30 psu). The fish were fed a commercial diet (1.5 or 2.0 mm pellets; crude protein 51%, crude fat 12%, digestible energy 18.0 MJ; Aller Nova, Aller Aqua, Christiansfeld, Denmark) twice daily at 9 a.m. and 4 p.m.
Experimental groups and management
Sixteen small net cages (1.5 × 0.9 × 0.9 m) were installed on eight rectangular FRP tanks (5 × 1 × 1.2 m) designed for stable juvenile (two net cages/tank). One unit was used for each experimental temperature: 19, 23, 27, and 31°C. Each unit consisted of two tanks (four net cages) to perform a duplicate experiment and one sand filter (MFS-600, Sam Ji Tech, Anyang, Korea) connected with PVC pipes (diameter 50 mm), and all water was replaced with new water at the experimental temperature every day or once every two days. In each tank, juvenile RGGG were stocked in one net cage and juvenile RG were stocked the other net cage in a same tank. The fish were randomly selected and stocked 30 fish/net cage (two experimental grouper/temperature/breed line). In the four groups, the size of the juvenile RGGG (SL 7.54±0.01–7.69±0.01 cm, BW 12.39±0.10–12.61±0.18 g) was significantly larger than that of the juvenile RG (SL 6.57±0.02–6.68±0.01 cm, BW 7.69±0.05–8.16±0.09 g) (p < 0.05), which could be attributed to a difference in growth from hatching to beginning of the experiment (five months). The experimental fish were stocked at 23°C, which was then increased by 4°C/day for the 27°C and 31°C group. For the 19°C group, low room temperature (10–15°C) was used to decrease the water temperature by 2°C/day. A digital temperature controller connected to a submersible titanium heater (2 kW, Sam Ji Tech, Anyang, Korea) was installed at the bottom of each tank to regulate and maintain the water temperature during the experimental period. The experiment was performed for eight weeks from the day the experimental fish were stocked, including the adjustment period, during which pellets (1.5, 2.0, and 3.0 mm pellets) from Aller Nova were provided manually until satiation, as during the acclimation period. Satiation was defined by a marked drop in feeding activity. The pellets were provided first to one of the net cages in each tank; uneaten pellets that sank to the bottom of the tank were collected and counted after pellet provisioning by siphoning. Afterwards, pellets were provided to the remaining net cage in the tank, and siphoning was repeated. The pellets collected by siphoning were excluded from the overall weight of the provided pellets. Sea water at the experimental temperature was supplied to compensate for the loss of rearing water from backwashing, rinsing, siphoning, and evaporation. During the experimental period, dissolved oxygen was provided through a pure oxygen generator (Aqua 7 L, Oxus Korea Co., Ltd., Seoul, Korea) and air blower (ASP140, Toshipump, Gwangju, Korea) to maintain an oxygen concentration above 5 mg/L. Salinity was maintained at 25–30 psu, the same as natural seawater.
Growth analysis and statistics
Each individual’s initial, two-week, five-week, and final (eight-week) body weight (BW) was measured with an electronic balance (MW-IIN, CAS Co., Ltd., Yangju Korea) to the nearest 0.01 g, and standard length (SL) measurements were taken with digital vernier calipers (500 series, Mitutoyo Co., Kawasaki, Japan) to the nearest 0.01 cm. Experimental fish did not receive pellets for one day preceding the measurements and were anesthetized just before the measurement using 200–300 ppm Triciane-S (Syndel USA, Ferndale, Washington, USA). The growth parameters were calculated according to the measurements: specific growth rate (SGR) = 100 × [ln final body weight (g) - ln initial body weight (g)] / feeding day (Lugert et al. 2016); weight gain (WG) = 100 × [final body weight (g) - initial body weight (g)] / initial body weight (g) (Bunlipatanon and U-Taynapun 2017); condition factor = 100 × body weight (g) / standard length (cm)3 (Fulton 1904); food consumption (FC) = food consumed (g) / day (Pérez-Casanova et al. 2009); and feed conversion ratio (FCR) = total food consumed (g) / body weight gain (g) (Lupatsch et al. 2010). The FCR calculation included the weight of dead individuals.
A nonlinear regression analysis was used to determine the optimum temperature for SGR and FCR for juvenile RG and RGGG. Data on growth parameter were presented as mean±SE and checked for normal distribution and equality of variances prior to statistical analysis. One-way ANOVA was used to test for significant difference; Tukey’s honest significance difference test was conducted when equality of variances was met, while the Games-Howell test was used when equality of variances was not met (IBM SPSS Statistics Version 21 program, SPSS Inc., Chicago, IL, U.S.). Statistical significance was verified at p < 0.05.
3. Results
All individuals survived over the eight weeks except for five individuals in the 31°C RGGG group, which died of cannibalism between weeks five and eight. RGGG growth sped up significantly as water temperature increased (p < 0.05, Fig. 1, Table 1). Significant differences in size (SL and BW) between the RGGG groups emerged starting on week two (p < 0.05, Fig. 1). At week eight, the size (SL 13.29±0.05 cm, BW 70.53±0.52 g, Fig. 1), SGR (3.41± 0.03%/day), and WG (469.45±8.67%, Table 1) were significantly highest in the 31°C group (p < 0.05). Compared with growth between the RG groups, aside from the 19°C group, RG only demonstrated a significantly higher BW in the 27°C group (21.66±0.35 g) (p < 0.05, Fig. 1). SGR and WG in RG were highest in the 27°C group (1.99± 0.07%/day and 176.05±10.12%, respectively), followed by 31, 23, and 19°C (p < 0.05, Table 1). At the same water temperature, SGR and WG between the two breeding lines were only significantly different at higher temperatures (27 and 31°C), and these parameters of RGGG in the 27°C and 31°C group had significantly greater than those of RG in the 27°C group having the largest values among the RG groups (p < 0.05). According to the regression curve analysis, the optimum temperature for SGR within the range of experimental temperature was 31.0°C for RGGG and 27.5°C for RG (Fig. 2a).
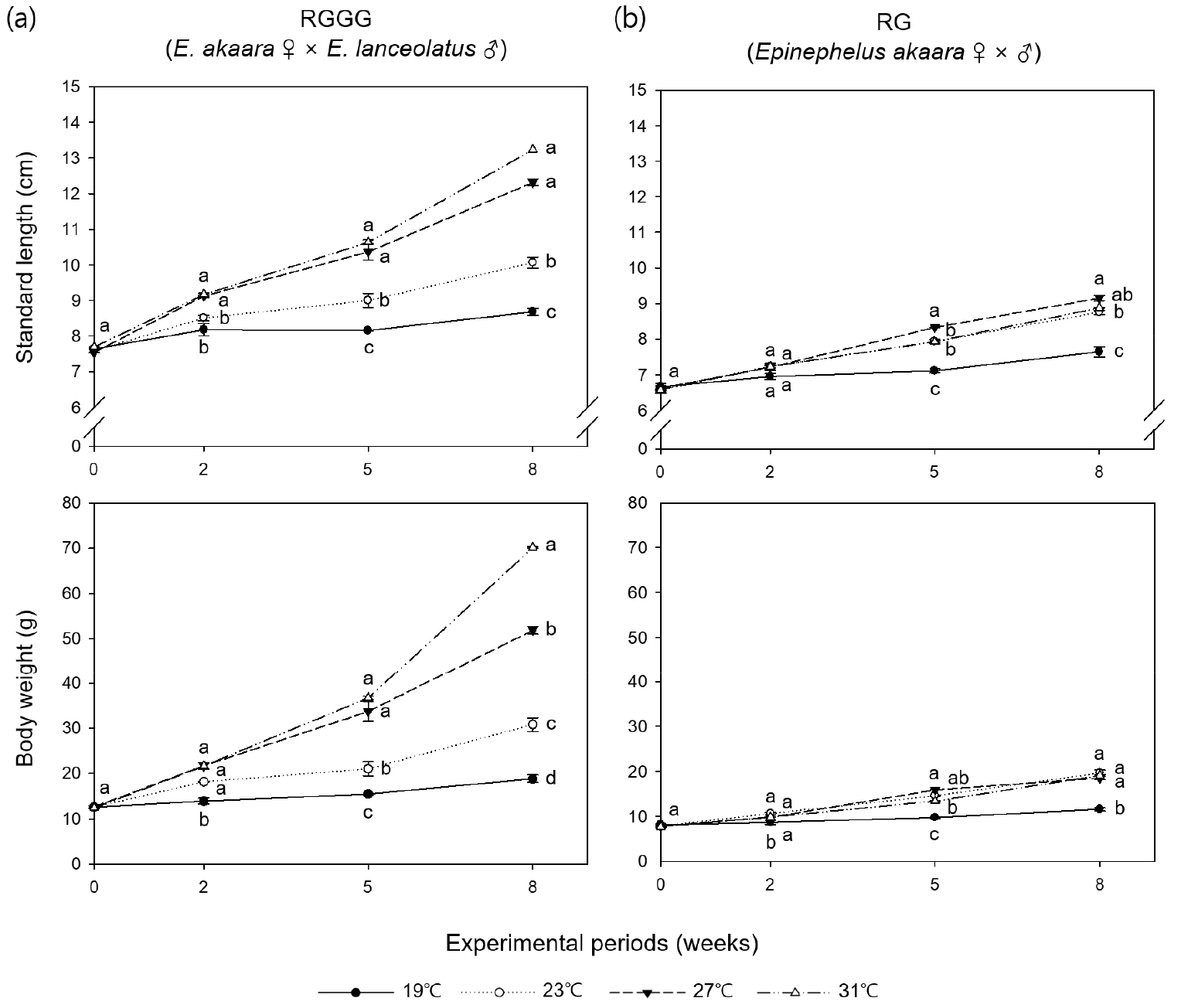
Fig. 1.
Standard length (cm) and body weight (g) of (a) juvenile RGGG (Epinephelus akaara ♀ × E. lanceolatus ♂) and (b) juvenile RG (E. akaara ♀ × ♂) reared for eight weeks at four different water temperatures. Different lowercase letters indicate the results of one-way ANOVA and Tukey’s honesty significant difference test when equality of variances was met; otherwise, the Games-Howell test was used. Values with the same letter are not significantly different at the 0.05 level. Grouping information for experimental periods of standard length and body weight of juvenile RGGG at eight weeks and initial body weight of juvenile RG is based on the Games-Howell test
Table 1.
Growth performance of juvenile RGGG (Epinephelus akaara ♀ × E. lanceolatus ♂) and juvenile RG (E. akaara ♀ × ♂) reared for eight weeks at four different water temperatures
Letters after values (mean±SE) indicate the results of one-way ANOVA and Tukey’s honesty significant difference test when equality of variances was met; otherwise, the Games-Howell test was used. Grouping information for condition factor is based on the Games-Howell test. Values with the same letter are not significantly different at the 0.05 level.
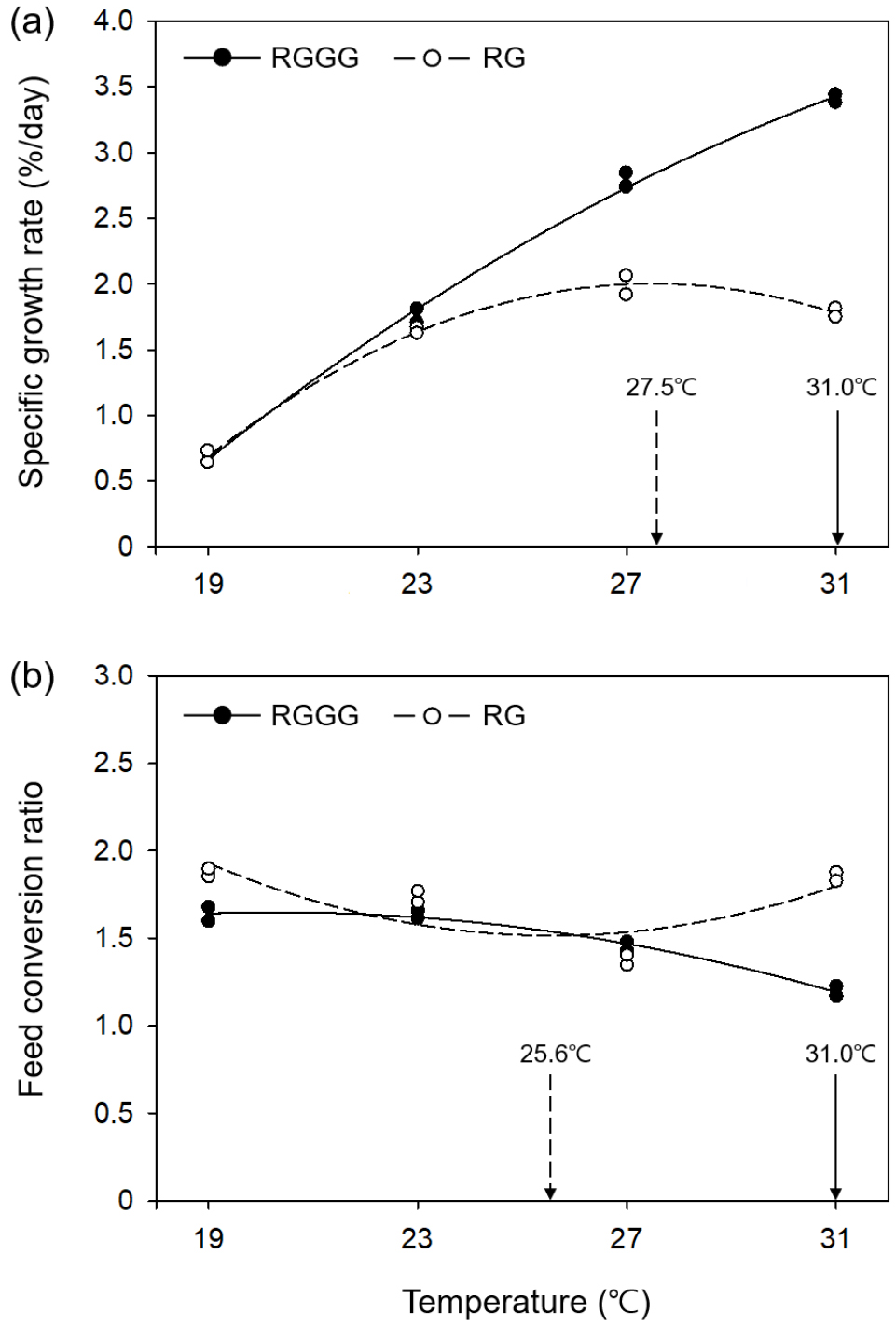
Fig. 2.
Changes in specific growth rate (a) and feed conversion ratio (b) across water temperature for juvenile RGGG (Epinephelus akaara ♀ × E. lanceolatus ♂) and juvenile RG (E. akaara ♀ × ♂) after eight weeks. The curves represent the least-squares second order polynomial fit to the data: SGR and FCR = aT2 + bT + c where SGR = specific growth rate; FCR = feed conversion ratio; T = temperature; and a, b, and c are constants determined by the nonlinear regression. (a) SGRRGGG = -0.0071T2 + 0.5838T - 7.8747 (R2 = 0.9984). SGRRG = -0.0183T2 + 1.005T - 11.8071 (R2 = 0.9998). (b) FCRRGGG = -0.004T2 + 0.1623T + 0.0019 (R2 = 0.9958). FCRRG = 0.0096T2 - 0.4906T + 7.7868 (R2 = 0.6485)
There was no significant difference in condition factor at the different water temperature of the same breeding line (p > 0.05, Table 1). However, when the two breeding lines were compared at the same water temperature, the condition factor of RGGG was significantly higher than that of RG for the 23°C and 31°C groups (p < 0.05).
Both the breeding lines demonstrated an increase in FC with an increase in temperature, hence the highest FC was in the 31°C group (p < 0.05, Table 1), however, there was no significant difference between 23°C and 27°C group, and 27°C and 31°C group in RG.
FCR in RGGG decreased at higher temperatures, thus was significantly lowest in the 31°C group (1.20±0.03) and significantly highest in the 19°C and 23°C groups (p < 0.05, Table 1). In comparison, the FCR of RG significantly decreased when the temperature went up from 19 to 27°C (1.88±0.02 to 1.38±0.03), and then significantly increased in the 31°C group (1.85±0.02) (p < 0.05). When comparing the two breeding lines at the same temperature, the FCR of RGGG was significantly lower than that of RG in all temperature groups except the 27°C group (p < 0.05, Table 1). Additionally, the FCR of RGGG in the 31°C group was the lowest throughout all water temperature groups of the two breeding lines (p < 0.05). According to the regression curve analysis, the optimum temperature within the experimental temperature range for FCR was 31.0°C for RGGG and 25.6°C for RG (Fig. 2b).
4. Discussion
The giant grouper used for hybridization in this study is a very fast-growing species that reaches body weights of 3 kg by the first year and 20 kg by the fourth year after fertilization (Sadovy et al. 2003; Vatanakul et al. 1995); it is often used as the paternal species in hybridization efforts aimed at enhancing growth in groupers (Chen et al. 2018; Ch’ng and Senoo 2008; Kiriyakit et al. 2011; Tian et al. 2015). Bunlipatanon and U-Taynapun (2017) reared three-month-old juvenile tiger grouper ♀ × giant grouper ♂ hybrid (initial mean BW 3.6 g) for ten weeks at 28°C, and reported that the hybrid grouper had higher SGR and WG than did the maternal purebred. This coincides with the observation in this study that juvenile RGGG grew faster than juvenile RG. In previous studies, the optimum temperature for the growth of juvenile tiger grouper ♀ × giant grouper ♂ was 26–30°C (initial mean BW 194 g, De et al. 2016) and 32.4°C (initial mean BW 121.62 g, Zhang et al. 2018). The red-spotted grouper used in this study for hybridization with giant groupers inhabit lower water temperature than do the tiger grouper used in the aforementioned study (preferred habitat temperature in nature: 28.2°C). Nonetheless, the optimum temperature for the growth of juvenile RGGG in this study and that of the juvenile tiger grouper ♀ × giant grouper ♂ in the previous study did not vary. Moreover, there was no significant difference in the SGR and WG between juvenile RGGG and RG at lower temperatures (19 and 23°C), but RGGG demonstrated significantly greater SGR and WG values than did RG at higher temperatures (27 and 31°C) (p < 0.05). Thus, considering the preferred water temperature of the giant grouper (28.1°C), the improved growth performance of juvenile RGGG in this study can be attributed to the effects of the hybridization with giant groupers.
There was no difference in optimum temperature for growth performance between juvenile RGGG and other groupers in tropics/subtropics, such as the orange-spotted grouper (E. coioides; 31.4°C, initial mean BW 14.75–281.41 g, Lin et al. 2008) and the Nassau grouper (E. stratus; 28–31°C, initial mean BW 3.20 g, Ellis et al. 1997). The growth of fish increases as water temperatures increase until it reaches the peak growth temperature, after which point growth is retarded as it nears the upper lethal temperature (Jobling 1997; Woiwode and Adelman 1991). The growth of the tiger grouper ♀ × giant grouper ♂ hybrid was lower at 34°C than 30°C (De et al. 2016) and that of the orange spotted grouper was lower at 35°C than 30°C (Lin et al. 2008). Thus, had there been a wider range of experimental temperatures in this study, the optimum temperature for juvenile RGGG growth would have been more accurately obtained from the regression curve between SGR and water temperature. However, since the natural temperature of the seawater in which net cages for rearing grouper is located are under 32°C even in the warm water region (Lin et al. 2008), it is unlikely that it can be applied to RGGG farming even if its optimum temperature for growth is higher than 31°C.
Condition factor is a non-lethal morphometric index that can be used to evaluate current body condition and the future growth of fish (Robinson et al. 2008). This index works under the assumption that a heavier individual at a given length has a more favorable body condition, which is supported by research on the relationship between the morphological index and physiological condition in many types of fish, such as the Atlantic salmon (Sutton et al. 2000). In this study, there was no difference in condition factor between groups in the same breeding line. The condition factor of juvenile RGGG was significantly higher than that of juvenile RG in 23°C and 31°C groups (p < 0.05), where there was no significant difference within each breeding line. This can be understood as a difference in intrinsic body shape resulting from the introduction of giant grouper traits rather than a difference in body condition between the two breeding lines. Especially, the condition factor of the juvenile RGGG in the 19°C group showed growth and survival without significant differences from the other groups. The survival rate of the juvenile tiger grouper ♀ × giant grouper ♂ hybrid (initial mean BW 121.62 g) was under 60% after 30 days of rearing at 19°C (Zhang et al. 2018). Thus, the difference in survival rates at low water temperatures between the two studies can be attributed to maternal traits and indicate the ability of juvenile RGGG to be reared at low temperatures, which is favorable to the aquaculture industry in temperate regions.
Protein digestibility enhances growth and increases with temperature (Mazumder et al. 2018; Muyan et al. 2006). The juvenile RGGG in this study showed increased FC and utilization at higher temperatures, which influenced the difference in growth depending on water temperature. When the water temperature is higher than the optimum temperature for growth, however, dietary energy is expended as basal metabolic energy, which retards growth by diminishing the feed used for growth (De et al. 2016; Jobling 1997). This corresponds to the results of this study, in which the FCR of juvenile RG decreased with increasing temperature, reaching its lowest value in the 27°C group, which was the optimum temperature for SGR, then increasing in the 31°C group despite having the same FC as the 27°C group. This also corresponds with results obtained in the tiger grouper ♀ × giant grouper ♂ hybrid (De et al. 2016; Zhang et al. 2018) and Nassau grouper (Ellis et al. 1997).
It is understood that the optimum temperature for FCR in fish is lower than the optimum temperature for growth when food is not limited (Jobling 1997). This coincides with regression curve analysis results from this study, in which the optimum temperature for FCR in juvenile RG (25.6°C) was 1.9°C lower than that for SGR (27.5°C). This tendency was also observed in hybrid bass (Morone saxatilis ♀ × Morone chrysops ♂): 21.2°C vs. 26.8°C (Woiwode and Adelman 1991), Atlantic salmon (Salmo salar): 9.4°C vs. 13.0°C (Handeland et al. 2008), and turbot (Scophthalmus maximus): 17.4°C vs. 19.6°C (Imsland et al. 2001). Nonetheless, although the optimum temperature for SGR and optimum temperature for FCR in juvenile RGGG were both observed at the highest experimental temperature of 31°C, the tendencies described by Jobling (1997) may have been observed if the experimental temperature was higher.
In conclusion, the growth performance of juvenile RGGG differed from that of juvenile RG in this study, in which growth and feed utilization increased dramatically as the water temperature increased from 19 to 31°C. The optimum temperature of juvenile RGGG to support their rapid growth performance was demonstrated to be similar to that of the paternal fish species (giant grouper), not the maternal fish species (red-spotted grouper). Additionally, juvenile RGGG had WG that was 2.7 times (31°C) and 1.8 times (27°C) greater than juvenile RG in the 27°C group during the eight-week experimental period, indicating a potential for shortened production time and enhanced quantity, which is expected to improve productivity in the aquaculture industry. With regards to the growth performance of juvenile RGGG, this novel hybrid grouper is not only appropriate for rearing in the tropics/subtropics, but also the temperate regions, where the hot effluent water from power plants (approximately 7–9°C warmer than natural sea water) and ground water (maintained at 28°C throughout the year) can be used as sources of energy. To improve the productivity and marketability of this hybrid, we plan to conduct research on the development of rearing techniques suitable for rapid growth on an industrial scale, disease resistance such as Vibrio spp. and expression of red body color resembling maternal fish species.




