1. Introduction
2. Materials and Methods
Sampling efforts
Histology
Enzyme-linked immunosorbent assay (ELISA)
Total protein, carbohydrate, and lipid content analysis
3. Results
M. chungmuensis infection in the oyster eggs
Reproductive stage of the M. chungmuensis infected oysters
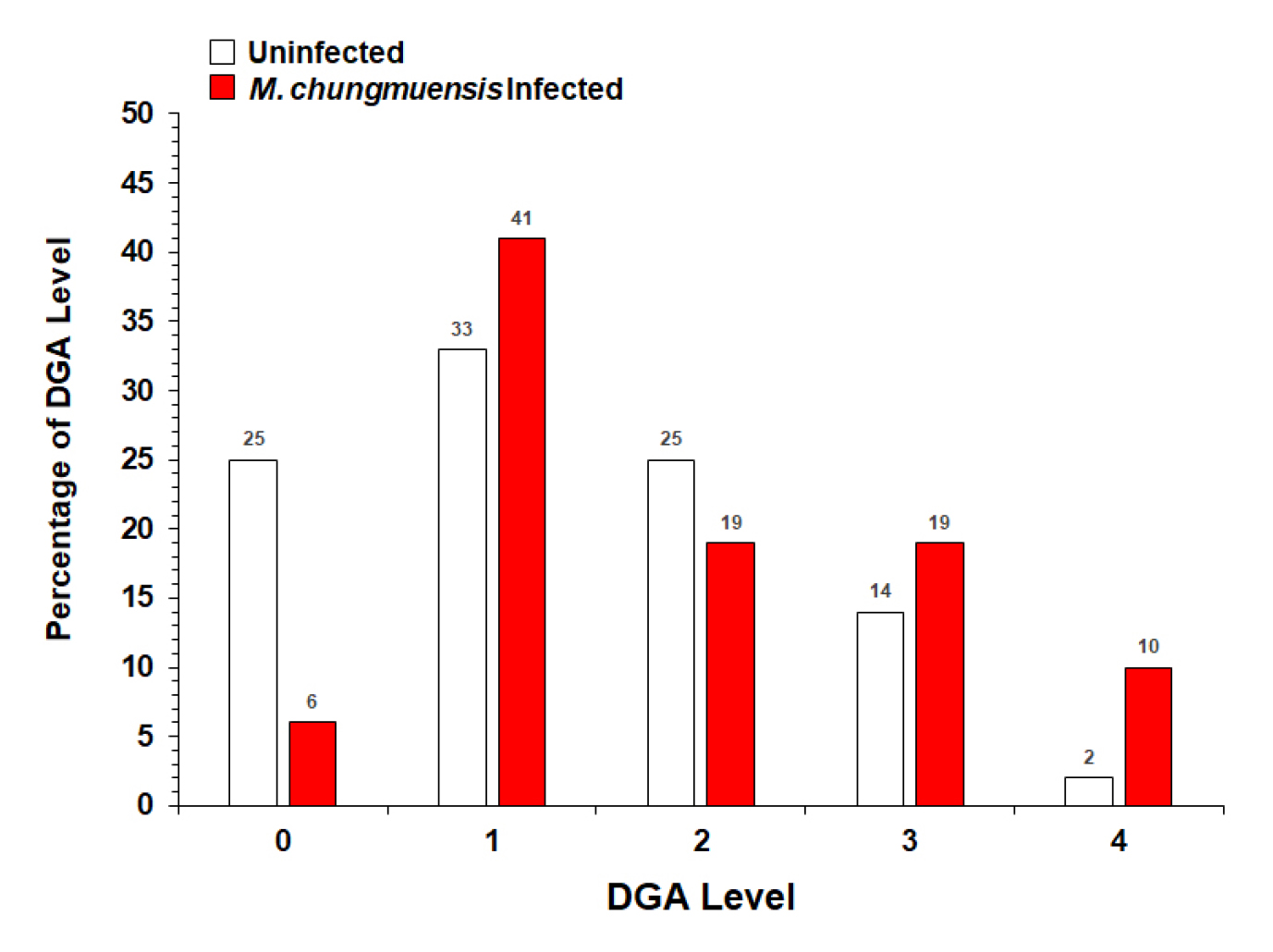
Digestive Gland Atrophy (DGA)
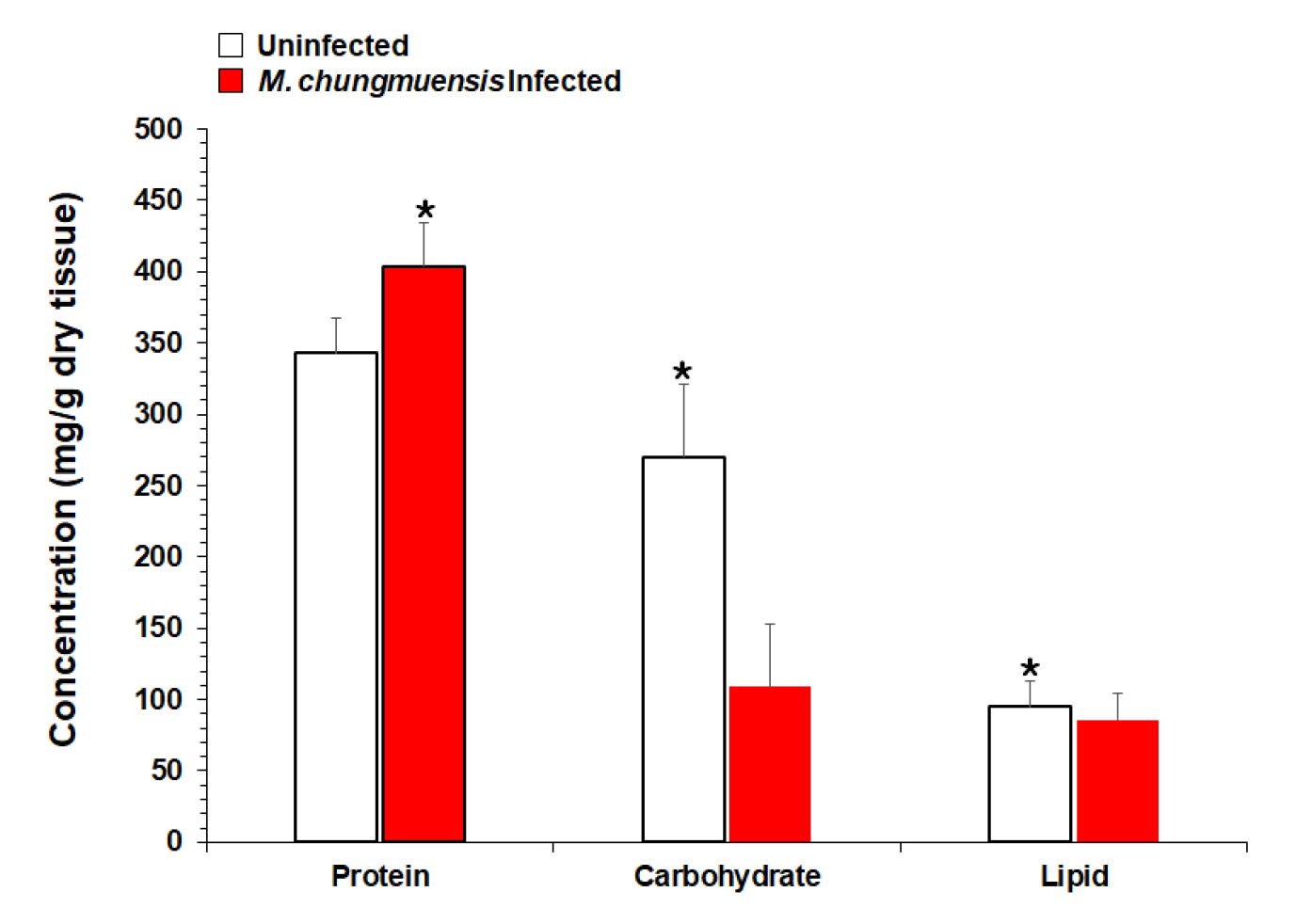
Biochemical composition of the tissue
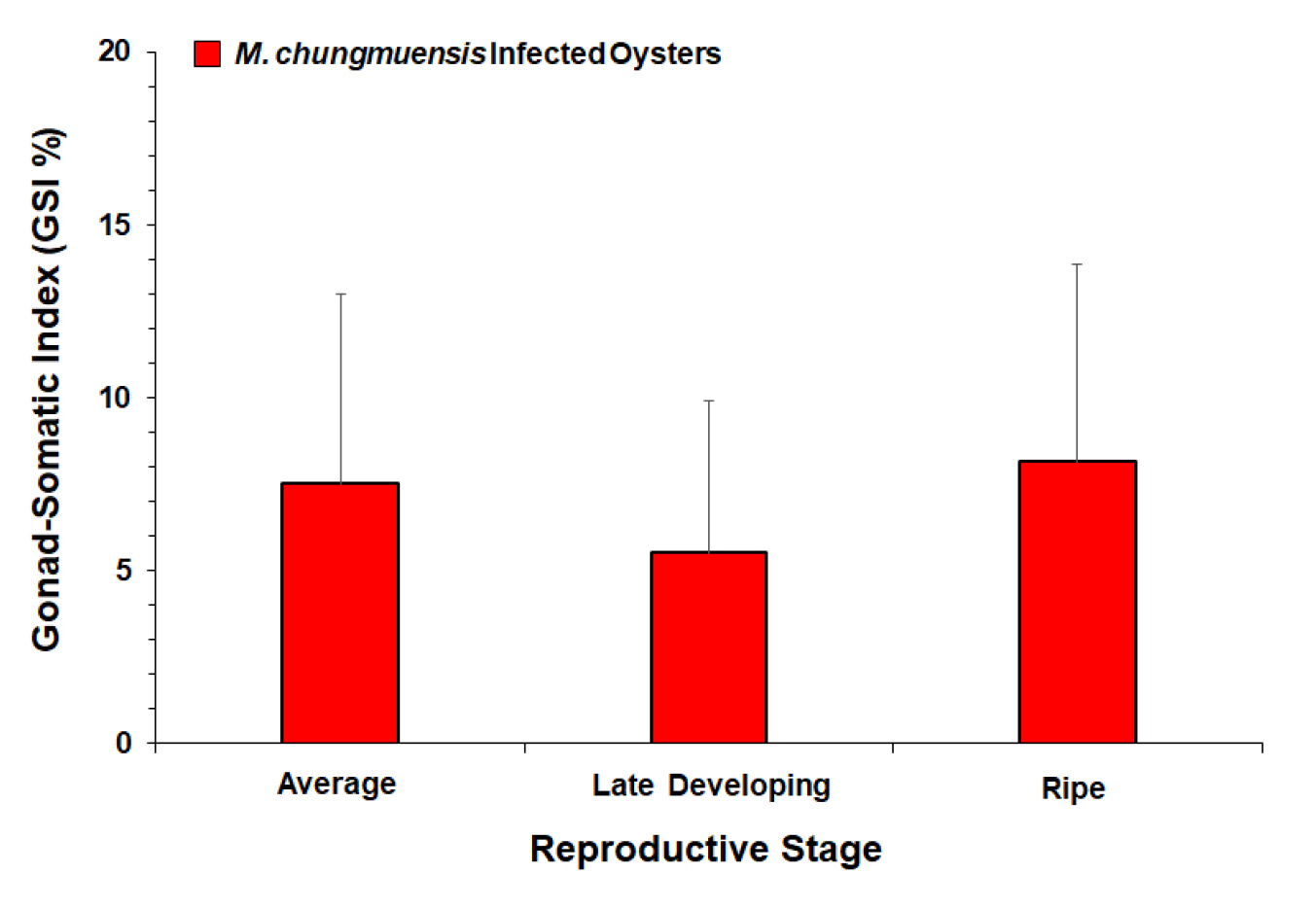
Reproductive effort of M. chungmuensis infected oysters
4. Discussion
Ecology and epidemiology of M. chungmuensis
Effects of M. chungmuensis parasitism on the female oysters
1. Introduction
Native to the North-East Pacific Ocean, the Pacific oyster Crassostrea gigas plays a crucial role in the shallow intertidal and subtidal ecosystem, linking the primary production to the upper trophic level. On the south coast of Korea, where the coastline includes numerous semi-enclosed bays, the Pacific oysters are cultured extensively in the middle of the bays using floating long lines (see the review of Choi 2008). The Pacific oysters are known to be resistant to marine epidemic diseases caused by microscopic organisms, whereas other species of oysters in aquacultures, such as C. virginica on the east coast of the United States and Ostrea lurida in the European waters, have been suffering from various bacterial and protistan diseases such as perkinsosis and marteiliosis (see the review of Degremont et al. 2015).
In the endemic area of the Pacific oyster in Korea and Japan, the Pacific oysters have been suffering from a unique protozoan parasite, Marteilioides chungmuensis, which infects the cytoplasm of the mature oocytes (Comps et al. 1986; Comps 1987; Park and Chun 1989). In the mature oocytes, the ovarian oyster parasite M. chungmuensis develops into primary, secondary, and tertiary cells and finally differentiates into a sporont (Park and Chun 1989; Limpanont et al. 2013). According to Itoh et al. (2004), the early developing stage of M. chungmuensis can be found in the epithelial tissue of the gills, mantle, and labial palps, as they are believed to be liberated from the infected oocytes. The ovarian oyster parasite often exhibits enlarged and deformed ovaries, which form multiple yellowish nodules on the mantle (Ngo et al. 2003; Limpanont et al. 2013). Several studies have reported that the infected female oysters fail to spawn during the spawning season, and the unreleased infected mature eggs are retained as late as early winter (Park et al. 1999, 2003; Tun et al. 2007, 2008; Ngo et al. 2003). Such retained eggs are finally phagocytosed by the oyster hemocytes and absorbed, although the amount of the infected eggs during the post-spawning period is yet to be determined. According to Tun et al. (2007), M. chungmuensis infected oysters in Miyagi Prefecture, Japan, spawned continuously from October to March, suggesting that the infected oysters released the eggs into the environment during the non-spawning period.
According to the studies carried out on the south coast of Korea, the Pacific oysters initiate gametogenesis in early spring (January and February) after a short period of resting in late fall to winter (Ngo et al. 2002; Kang et al. 2003, 2010). As the sea surface temperature increases fast from April to May, the female oysters transform the stored energy into eggs and are ready to spawn in early summer (June and July). Throughout spawning, the females discharge a vast amount of eggs into the environment; according to Kang et al. (2003), the female oysters discharge eggs that account for 42% of their body weight through spawning. Unlike normal oysters, M. chungmuensis infected females may perish a substantial amount of eggs in an annual reproductive cycle as the infected females fail to spawn. The quantity of the perished eggs by M. chungmuensis parasitism is an ecologically interesting issue, although the quantity is yet to be determined.
Using histology and an indirect enzyme-linked immunosorbent, we measured the metabolic contents, level of digestive gland atrophy, and the quantity of eggs in M. chungmuensis-infected oysters from Tongyoung on the south coast collected in December 2010. Accordingly, we report the nutritional condition and the quantity of the perished eggs caused by the ovarian parasites (100 uninfected and 100 infected oysters), which provide insight into the paramyxean parasitism on the host reproduction and metabolism.
2. Materials and Methods
Sampling efforts
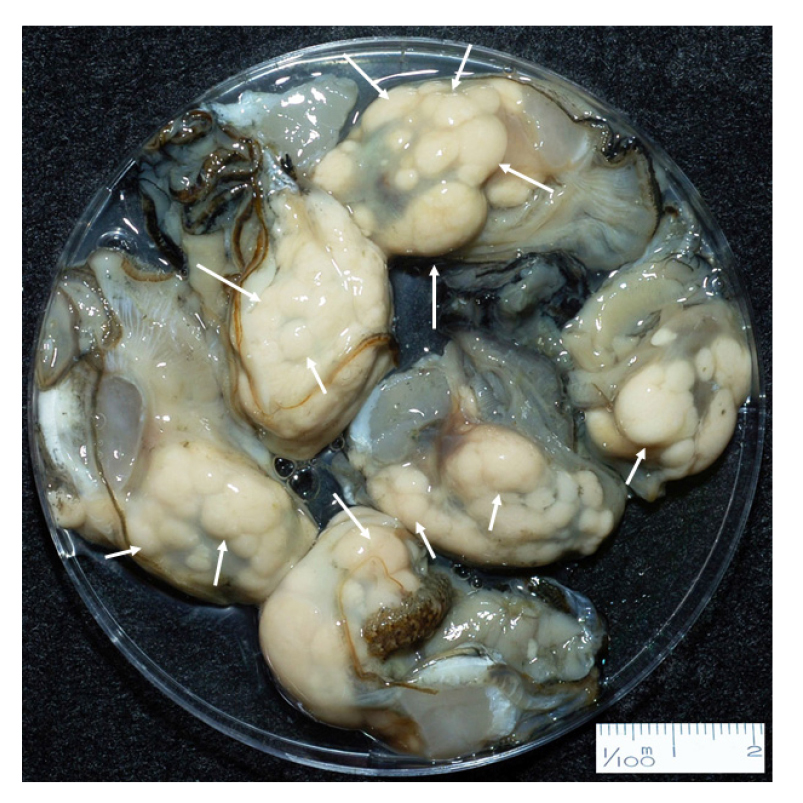
Located on the south coast of Korea (Fig. 1), small bays in Tongyoung include numerous oyster farms, where the Pacific oysters are cultured using suspended long-lines. In the bays, the hardened seed oysters are transplanted in May, and then the oysters reach their marketable sizes of 7–8 cm in shell length (SL) in late fall and early winter. The harvested oysters in December and early January (Choi 2008) are often waterly and exhibit eccentric lumpy nodules on the body as a sign of M. chungmuensis infection (Fig. 2).
For the investigation, one hundred uninfected and M. chungmuensis-infected oysters were supplied from Tongyoung in December 2010. A three mm-thick section was cut dorso-ventrally in the middle of the body and fixed in Bouin's fixative. The remaining tissues were lyophilized and homogenized using mortar and pestle for ELISA and the total protein, lipid, and carbohydrate in the tissues.
Histology
For histology, the tissues containing gonads were dehydrated and embedded in paraffin. Paraffin blocks were sectioned at 6 µm thickness, stained with Harris's hematoxylin, and counterstained with eosin Y. Under a light microscope, The reproductive stage of the uninfected and infected oysters was categorized, according to Mondol et al. (2012), as resting, early developing, late developing, ripe, partially spawning, and spent. The nutritional condition of oysters was determined from histology by grading the digestive gland atrophy (DGA) level characterized by thinning of the lumen and epithelium of the digestive tubules (Kang et al. 2010). Based on the microscopic appearance of the digestive tubules, the DGA of each oyster was graded from 0 (least level of DGA) to 4 (highest level of DGA, see Fig. 3), according to Winstead (1995) and Kang et al. (2010).
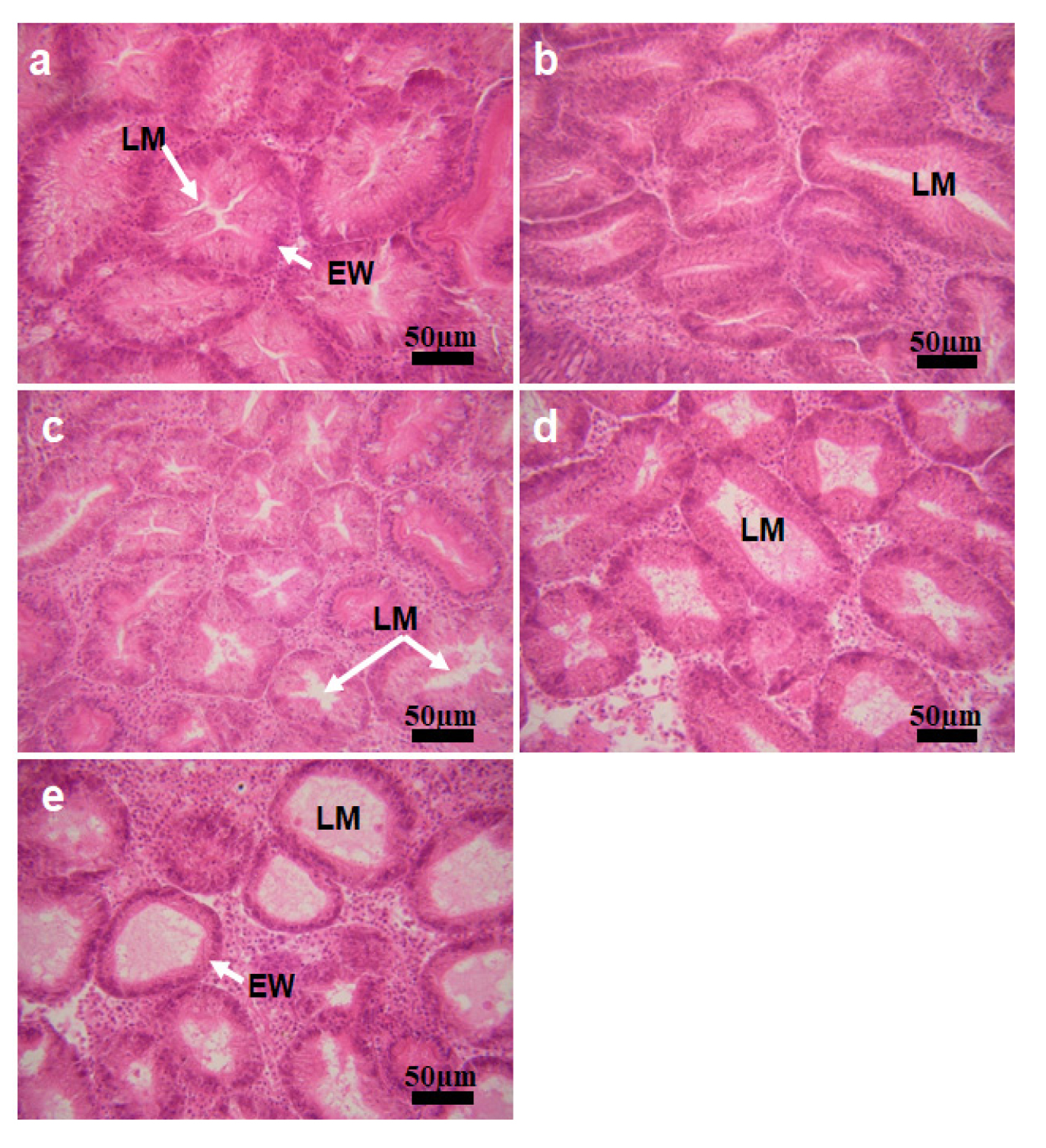
Fig. 3.
Micrographs showing different levels of the digestive gland atrophy (DGA) a, DGA score 0, as the area of the lumen (LM) is minimal. EW, epithelial wall of the digestive tubule. b, DGA 1, as the area of the lumen expands compared to the score 0. c, d, DGA 2 and 3, as the LMs are expanded wider. e, DGA 4, the highest atrophy condition
Enzyme-linked immunosorbent assay (ELISA)
Indirect ELISA was applied to measure the quantity of the oysters' eggs in the infected oysters, in which the rabbit anti-oyster egg protein IgG served as the primary antibody (Kang et al. 2003; Ngo et al. 2006; Hong et al. 2022). For the assay, 20 mg of the freeze-dried and homogenized oyster tissue subsample was taken from each sample, dissolved in phosphate-buffered saline (PBS, 0.15M NaCl, pH 7.6), and further homogenized using an ultrasonifier. The homogenate was diluted to 500-fold to fit into the optimal antibody-antigen reaction in indirect ELISA. The indirect ELISA assay used in this study followed the protocol reported by Kang et al. (2003) and Park and Choi (2004). Based on the egg quantity measured from the indirect ELISA, the gonad-somatic index (GSI) was assessed as the ratio of the mass of eggs to the total body weight in dry weight.
Total protein, carbohydrate, and lipid content analysis
Total protein, lipid, and carbohydrate contents in each oyster used in histology and ELISA were determined. Total protein level was assessed using the method of Lowry et al. (1951) after hydrolyzing the tissues in 0.1 M NaOH at 37℃ for 2 hours. Bovine serum albumin was used as the standard in the protein assay. The phenol-sulfuric method by Dubois et al. (1956) was applied to determine the total carbohydrates, as dextrose anhydrase was included in the assay as the standard substance. Total lipids in the tissues were assessed gravimetrically by extracting the lipids using a mixture of chloroform and methanol (Bligh and Dyer 1959). The quantity of protein, carbohydrate, and lipid was expressed as mg/g dry tissue weight.
3. Results
M. chungmuensis infection in the oyster eggs
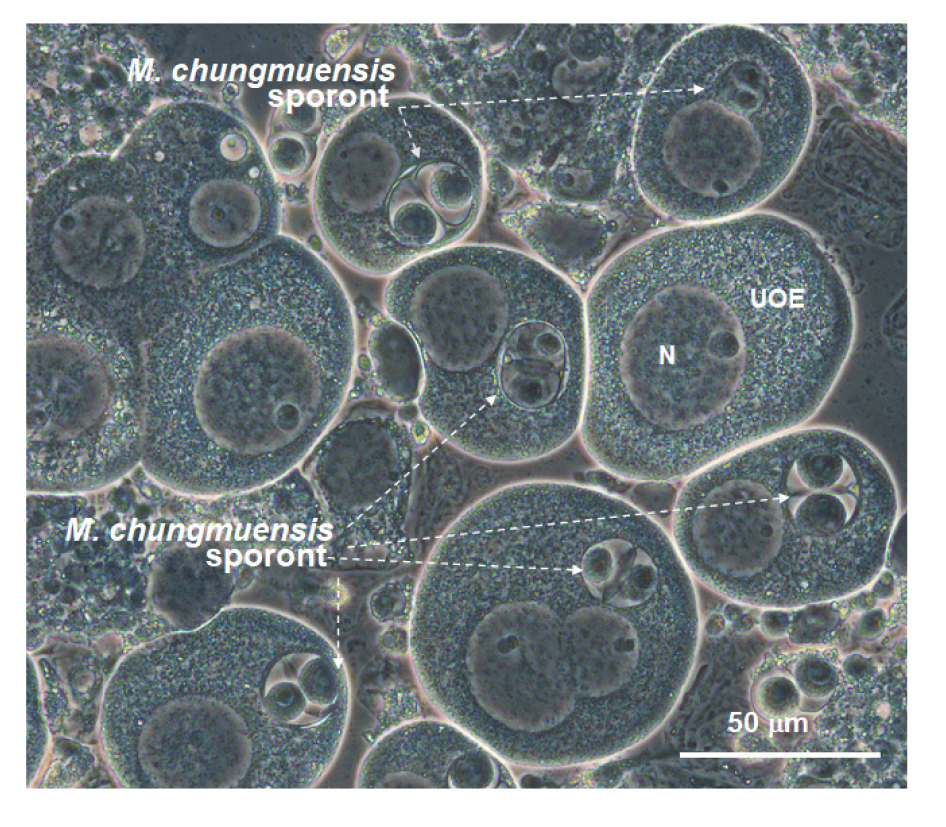
Fig. 4 shows a micrograph of M. chungmuensis sporonts in intact oysters eggs examined under the differential interference contrast (DIC) phase. Like other paramyxean parasites, M. chungmuensis sporonts exhibited a typical cell-inside-cell structure, mostly with two secondary cells in the sporonts.
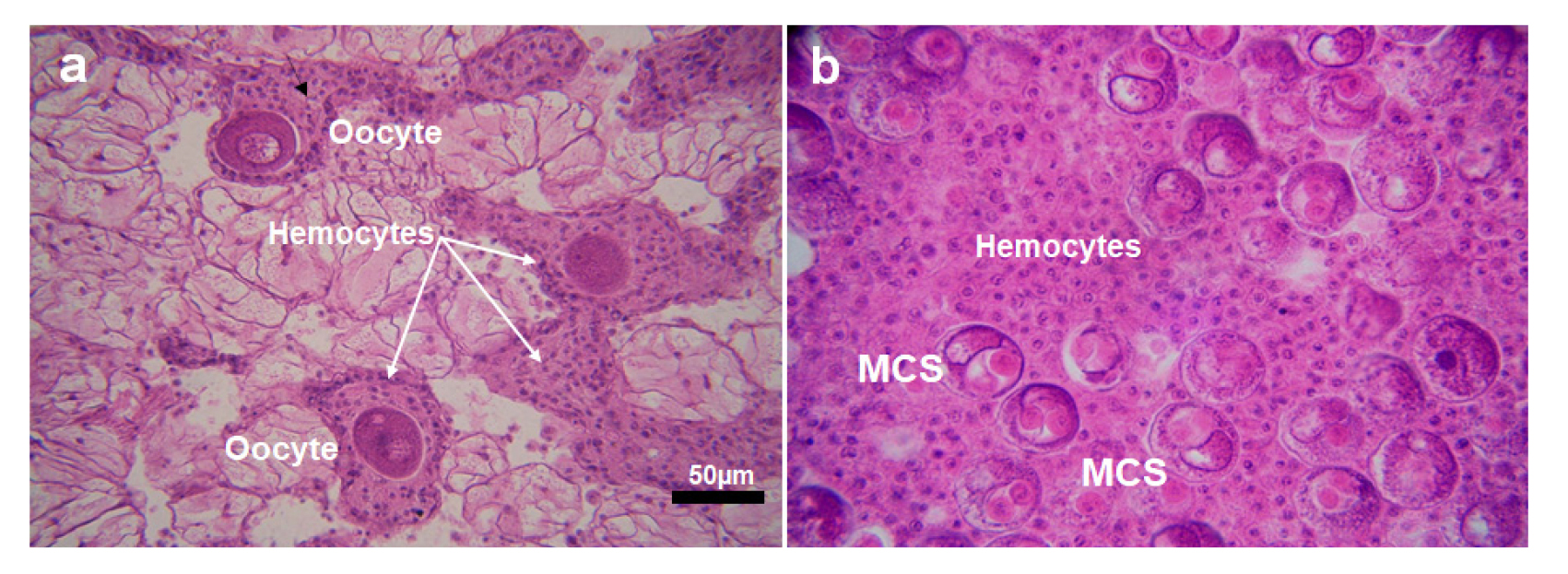
In December, some uninfected oysters exhibited eggs in resorption, characterized by massive hemocytes encompassing the eggs, a sign of auto-phagocytosis (Fig. 5a). In the infected eggs, sporonts containing one or two secondary cells were observed. The high density of hemocytes was also observed in the follicles of the infected females (Fig. 5b).
Reproductive stage of the M. chungmuensis infected oysters
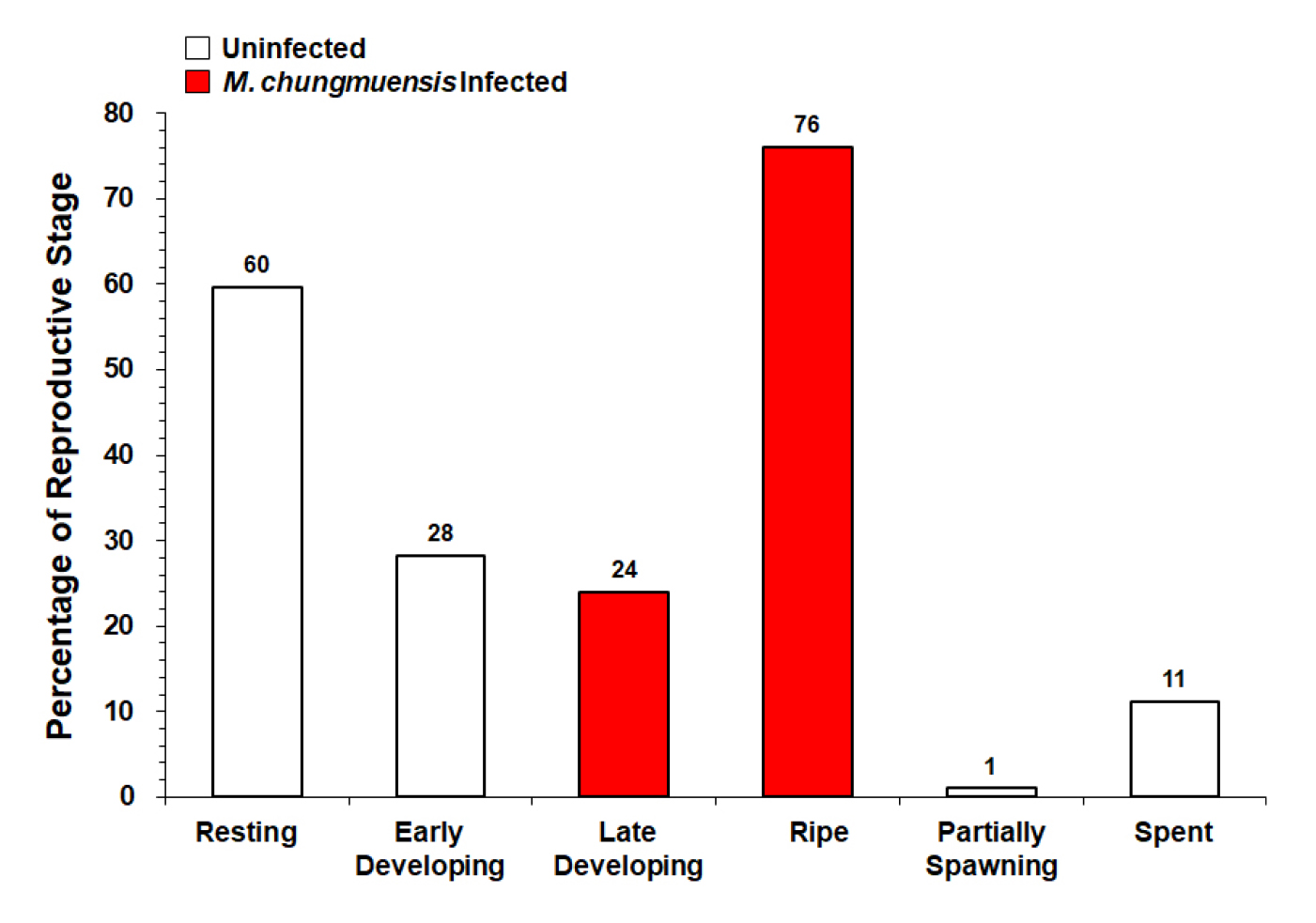
Fig. 6 plots the gametogenic condition of the female oysters collected from Tongyoung in December 2010. The histology revealed that 76% of the infected females were in the ripe stage, showing mature eggs with M. chungmuensis sporont in the cytoplasm and a few uninfected eggs. Notably, 24% of the infected females were in the late developing stage, characterized by a large number of small oocytes. Contrary to the infected, most uninfected oysters were in the resting (60%) and early developing (29%) stages. Some uninfected oysters (11%) were in the spent stage, exhibiting residual eggs phagocytosed by the hemocytes.
Digestive Gland Atrophy (DGA)
Histology indicated that in December, the DGA level of M. chungmuensis infected oysters was higher than the uninfected oysters (Fig. 7). Of the one hundred oysters examined by histology, 10% of the infected oysters were in DGA level 4, whereas only 2% of the one hundred uninfected oysters examined was in DGA level 4. Histology also indicated that the lowest DGA level (i.e., level 0) of M. chungmuensis-infected oysters was approximately four times lower (6%) than that of the uninfected oysters (25%).
Biochemical composition of the tissue
Protein was the major component of the uninfected oyster tissue, as protein accounted for 34.3% (343 mg/g dry tissue) of the total dry tissue weight, followed by carbohydrates 27.0% (270 mg/g dry tissue) and lipids 9.5% (95 mg/g dry tissue)(Fig. 8). In the infected oysters, the protein accounted for 40% (404 mg/g dry tissue) of the total dry weight, and the carbohydrate and lipid accounted for 11 (109 mg/g dry tissue) and 9% (86 mg/g dry tissue) respectively (Fig. 8). Student t-tests indicated that the total carbohydrate and lipid levels in the infected oyster tissues were significantly lower than those in the uninfected oysters (p < 0.05). In contrast, the total protein in the infected oysters was significantly higher than the protein in the uninfected (p < 0.05).
Reproductive effort of M. chungmuensis infected oysters
ELISA was performed limitedly on the infected oysters (N=96) as the most uninfected exhibited no eggs in the gonad. GSI of the M. chungmuensis infected oysters ranged from 0 to 30.4%, with an average of 7.5%. Fig. 9 plots the mean GSI of the infected oysters according to their reproductive stage, as GSI in the ripe stage (8.2%) was somewhat higher than the GSI in the late developing stage (5.5%).
4. Discussion
Ecology and epidemiology of M. chungmuensis
Eukaryotic single-celled protists are common in the shallow coastal environment, and some of the members form a symbiotic association such as parasitism with large metazoan animals, including mollusks, polychaetes, and crustaceans (See review of Keeling and Campo 2017). In particular, paramyxean protists parasitize some economically valuable marine bivalves, including oysters, mussels, and cockles, resulting in mass mortalities and subsequent economic losses (Berthe et al. 2004; Carrasco et al. 2015). According to the World Register of Marine Species database (WORMS), M. chungmuensis belongs to the Phylum Cercozoa, Class Ascetosporea, Order Paramyxida, and Family Marteliidae (https://www.marinespecies.org/aphia.php?p=taxdetails&id=562854).
The ovarian oyster parasite was first observed in the Pacific oysters raised in a small bay in Hiroshima, Japan, where oysters exhibited “abnormally enlarged ovary with a nodular appearance from autumn to winter” (Seki 1934, cited from Matsusato and Masumura 1981). According to Matsusato and Masumura (1981), such abnormal enlargement in the ovary phenomenon was common among the oysters cultured in subtidal suspended long-line and wild oysters in intertidal in Hiroshima. Imanaka et al. (2001) also reported a seasonality in the prevalence of ovarian oyster parasites, as oysters in Gokasho Bay in Mie Prefecture showed their highest in July at 52%.
On the south coast of Korea, the oyster ovarian enlargement phenomena were recognized in the 1970s from oysters on the south coast (Chun, 1972). Based on transmission electron microscopy of the sporont ultrastructure in the infected eggs, Comps et al. (1986) reported the oyster parasite as a new species, Marteilioides chungmuensis, by establishing a new genus. Imanaka et al. (2001) suspected that the pathogen responsible for ovary enlargement in Japan could be M. chungmuensis, reported in Korea. Itoh et al. (2002) also examined the ultrastructure of the micro-organisms involved in the enlargement of the oyster ovary in Okayama Prefecture, Japan, reporting that the pathogen responsible for the oyster ovary disease was M. chungmuensis.
Effects of M. chungmuensis parasitism on the female oysters
One of the sublethal impacts of M. chungmuensis infection in female oysters during the post-spawning season is markedly low glycogen or total carbohydrate content in the body, the energy reserve (Park et al. 2003). In this study, the total carbohydrate content in the M. chungmuensis-infected oyster tissues was approximately 2.5 times lower than in uninfected oysters. Similarly, Park et al. (2003) reported a significantly low level of glycogen in M. chungmuensis-infected Pacific oysters on the south coast of Korea, where the glycogen level of the infected oysters was approximately half of the uninfected oysters collected in September. They reported that such a low glycogen level or total carbohydrate content in oysters is a sign of poor nutritional conditions, possibly caused by a high level of parasitism (Park et al. 2003). To understand the nutritional condition of oysters, we also examined the degree of digestive tubule atrophy based on histology (Kang et al. 2010). Compared to the uninfected, M. chungmuensis-infected oysters showed comparatively higher levels of DGA, as the proportion of DGA level 4 (i.e., the highest atrophy level) of the infected oysters is 5 times higher than that of the uninfected. Accordingly, the DGA data indirectly suggest that the nutritional condition of the infected oysters is poorer than the uninfected oysters, possibly due to the M. chungmuensis parasitism.
Histology also revealed aggregation of the hemocytes along the infected oyster oocytes, suggesting possible pathogenicity of the parasite to the host organism (Fig. 5b). Infiltration of the hemocytes along the M. chungmuensis-infected oyster eggs was also observed from the infected oysters collected from the south coast during the post-spawning period (Park et al. 2003; Ngo et al. 2003). However, hemocyte infiltration was also observed in the uninfected oysters (Fig. 5a), indicating that hemocyte infiltration is a naturally occurring phenomenon in oysters in the spent stage. Ngo et al. (2003) and Kang et al. (2004) also reported a high density of hemocytes in the oyster follicles during the spent stage, as the unreleased residual gametes are reabsorbed through the phagocytosis by the hemocytes. According to Hong et al. (2020), the total hemocyte numbers in the giant honeycomb oyster Hyotissa hyotis in Jeju Island are increasing markedly during the spent stage, as the hemocytes are involved in reabsorbing the residual eggs by phagocytosis.
To understand the pathogenicity of M. chungmuensis infection in oysters, Choi et al. (2011) compared the number of total hemocytes, hemocyte types, and the phagocytosis capacity of M. chungmuensis infected oysters with normal oysters collected from the Tongyoung area on the south coast in August. The flow cytometry revealed that the total hemocyte counts in the infected oysters were significantly higher than in uninfected healthy oysters, and the granulocytes, the major type of hemocyte involved in phagocytosis in marine bivalves (Donaghy et al. 2009, 2010; Hong et al. 2013; Kim et al. 2020) are dominant in the infected oysters. Accordingly, a density of hemocytes observed from the infected oysters in this study is a sign of the pathogenicity caused by M. chungmuensis infection, as previously reported by Choi et al. (2011).
In the theories of life history evolution, the reproductive effort can be defined as “the proportion of the total energy budget of an organism devoted to the reproductive process” (Hirshfield and Tinkle 1975). According to Clark (1987), the reproductive effort of marine animals can be estimated by “measuring annual weight-specific gamete production (Pianka 1972)”. Practically, an annual reproductive effort of marine bivalves has been approximated by measuring the quantity of the gamete produced and indexed as the gonad-somatic index (GSI), which is a ratio of the weight of the gamete to the body weight (Bayne et al. 1983; Kang et al. 2003; Park and Choi 2004; Kim and Choi 2012; Uddin et al. 2007).
In this study, we estimated the amount of egg mas in the infected oysters using indirect ELISA (Kang et al. 2003; Hong et al. 2022). As histology indicated, most uninfected oysters were in the resting stages, exhibiting no germ cells in the gonad, whereas the infected female oysters contained fully mature eggs in the follicles. The GSI of M. chungmuensis infected oysters collected in winter was 7% (N=100), suggesting that the infected oysters retain mature eggs, which account for 7% of the body weight. Several studies have reported the reproductive effort of oysters estimated using indirect ELISA. According to Kang et al. (2003), Ngo et al. (2006), and Mondol et al. (2016), the GSI of oysters before spawning in summer reaches 30 to 40%. In the spent stage, the female oysters also retain a small number of residual eggs, which may account for 1–2% of the body weight (Kang et al., 2003; Ngo et al. 2006; Hong et al. 2022). Lee et al. (2019) also estimated the GSI of the Suminoe oysters in the spent stage in the Sumjin river estuary using indirect ELISA as 1–2%. Accordingly, the GSI of the infected oysters in December (7%) is unusual, suggesting that M. chungmuensis parasitism exerts substantial “reproductive loss” on the host. According to Tun et al. (2007), the retained eggs in M. chungmuensis-infected oysters are discharged via spawning during winter and early spring. As was reported in Japan (Tun et al. 2007), M chungmuensis infected oysters on the south coast of Korea may release the infected and uninfected but retained mature eggs during off spawning season in winter, although the oocytes are unlikely to succeed in fertilization and subsequent larval development.