1. Introduction
2. Material and Methods
Collection of macroalgal blades to set up clonal cultures of Ostreopsis sp. and Coolia canariensis
Growth Response of the Ostreopsis and Coolia Cultures to Temperature, Salinity, and Illuminance Gradients
Cytotoxicity of the Ostreopsis and Coolia cultures against Artemia Nauplii
Spatiotemporal distribution of Ostreopsis and Coolia species in Jeju Coastal Waters
3. Results and Discussion
Growth response to water temperature, salinity, and illuminance gradients by unialgal cultures of Ostreopsis sp. and Coolia canariensis
In situ spatiotemporal distribution of Ostreopsis and Coolia species in Jeju coastal waters
Vertical distribution of Ostreopsis and Coolia species in Moon-seom, an islet of Jeju Island
Cytotoxicity of the Ostreopsis sp. and Coolia canariensis strains against Artemia Nauplii
4. Conclusion
1. Introduction
In 2011, the occurrence and quantitative distribution of five epiphytic dinoflagellate (EPD) genera from Korean seas were first reported in the coastal waters of Jeju (Kim et al. 2011, 2021). EPD occurrence monitoring in Korean coasts (Baek 2012a; Jang 2013; Shah et al. 2013; Oh 2015; Park et al. 2020; Lee and Park 2020, 2022) was conducted in parallel with the strain-based studies on taxonomy and ecophysiology of EPD species (Jeong et al. 2012a, 2012b; Lee et al. 2014; Yoo et al. 2015; Shah et al. 2016; Jang et al. 2018). In addition, several new organic molecules were reported from the Ostreopsis cf. ovata strains of Jeju Island origin (Yih et al. 2019) through the search for novel marine natural products of mass-cultured EPD strains (Hwang et al. 2013, 2018; Yang et al. 2017; Lee et al. 2019, 2020).
Among the five EPD genera from Jeju coastal waters (Kim et al. 2011), the two genera, Ostreopsis and Coolia, including toxin producing species (Rhodes and Thomas 1997; Rhodes et al. 2000; Tibiriçá et al. 2020; Chomérat et al. 2022) commonly co-occur under bloom conditions (Gladan et al. 2019; Misurale et al. 2022). Therefore, a comparative study on the effect of the environmental variable on the growth rate and cytotoxicity of two strains representing the two genera, Ostreopsis and Coolia, in Jeju coastal waters would be worth conducting.
Epiphytic Ostreopsis species in the East Sea was first reported by Selina and Orlova (2010) from the Russian Ussuriiskii Bay. EPD species including two genera Ostreopsis and Coolia were later observed in the Jeju coastal waters (Kim et al. 2011; Jeong et al. 2012a, 2012b; Kang et al. 2013; Lee et al. 2013; Lim et al. 2013; Lee and Park 2020) and the East Sea (Baek 2012a, 2012b). Cytotoxicity of Ostreopsis species such as Ostreopsis cf. ovata and O. ovata isolated from Jeju coastal waters was identified and determined (Hwang et al. 2013, 2018; Shah et al. 2014) as was in the Ostreopsis spp. from Mediterranean (Gémin et al. 2020) and Adriatic seas (Gladan et al. 2019). Ciguatoxin-like toxin (Ostreopsis cf. ovata) and PSP toxin (Coolia monotis) were listed as the major toxins from 15 species of the alien/invasive Mediterranean microalgae (Marampouti et al. 2021). However, there are no literature reports on the toxicity of Coolia species inhabiting Korean seas (Yih et al. 2011; Lim and Jeong 2021). Coolia malayensis was the most prevalent and more toxic to larvae of Artemia and sea urchin than the other C. canariensis, C. tropicalis, and C. palmyrensis isolated from Hong Kong waters, where C. canariensis exhibited relatively higher toxicity to sea urchin larvae than that against Artemia larvae (Leung et al. 2017). Yessotoxin analogues, C56H78O18S2 and C58H86O18S2, were also determined in the Guam strain G6 of C. canariensis although no toxic effects of the water-soluble fraction of the strain on Artemia was observed (Phua et al. 2021).
Here, we compared the growth rate and biomass yield for the two toxic epiphytic dinoflagellate strains isolated from Jeju coastal waters, Ostreopsis sp. (strain KNUTP-OS 090203) and Coolia canariensis (strain CCJJ1; Jeong et al. 2012b) under the gradients of seawater temperature, salinity, and irradiance. Different cytotoxicity against Artemia larvae by Ostreopsis sp. and C. canariensis was also detected during the 72 h incubation experiments. At the same time, the contrasting in situ spatiotemporal distribution patterns of Ostreopsis spp. and Coolia spp. in Jeju coastal waters were considered to reflect the distinct and contrasting growth characteristics of the two EPD strains along the gradients of T, S, and irradiance.
2. Material and Methods
Collection of macroalgal blades to set up clonal cultures of Ostreopsis sp. and Coolia canariensis
A single cell of Ostreopsis on a blade of macroalga Gelidium amansii was isolated and established as a unialgal clonal culture (Ostreopsis sp. strain KNUTP-OS 090203). The macroalgal substrate was collected at a depth of 3 m off Aeweol (33°28′04.78" N and 126°19′23.69" E; with water temperature and salinity of 14.8°C and 33.7, respectively), Jeju Island in February 2009. The morphological characteristics of the Ostreopsis sp. strain were rarely distinguished from Ostreopsis cf. ovata HJ-2013 (GenBank accession number HE793379; Kang et al. 2013), but the SSU rDNA sequence of the Ostreopsis sp. was, however, clustered with Ostreopsis sp. ORUS clone- A1 (GenBank accession numbers KC848711 and KC99 1331; Efimova et al. 2014) rather than HJ-2013 (Hae Jin Jeong, personal communication). Therefore, taxonomic position of the Ostreopsis strain is set to a temporarily unidentified Ostreopsis species.
From a different macroalgal blade of the same Gelidium amansii sample above a unialgal clonal culture of Coolia canariensis (strain CCJJ1) was also established by isolating single cells (Jeong et al. 2012b). In the field, each macroalgal substrates was collected in situ using a plastic bottle, transported to the laboratory, shaken vigorously by hand over 100 times to separate the dinoflagellates, and finally filtered through a 500 μm Nitex mesh to obtain live dinoflagellate samples for single cell isolation. All clonal cultures were serially transferred every 3 weeks to bottles with f/2 media at a temperature of 20°C and a salinity of 30 under continuous illumination of 60 µmol photons m-2 s-1.
Growth Response of the Ostreopsis and Coolia Cultures to Temperature, Salinity, and Illuminance Gradients
The unialgal cultures of Ostreopsis sp. and C. canariensis were experimentally grown in triplicate 500-ml PC bottles for 34 days under the test gradients of temperature (15, 20, 25, 30, and 35°C), salinity (15, 20, 25, 30, and 35), and light intensity (10, 20, 50, 100, 200 and 300 µmol photons m-2 s-1) (Table 1). Unialgal inoculation for the experimental batch cultivation of the two strains was adjusted to obtain the initial target concentration of 50 dinoflagellate cells ml-1 in all the experimental bottles. A 5-ml subsample was collected daily from each bottle, and cell counting under a light microscope (Zeiss AxioCam HRc5, Carl Zeiss Ltd. Göttingen, Germany) was performed using a Sedgewick-Rafter counting chamber. Except for the respective environmental parameters (Table 1), the basic environmental conditions for all experimental cultures were 25°C and 30 salinity under continuous illumination with a cool-white fluorescent lamp at 50 µmol photons m-2 s-1.
The 6-day average daily specific growth rate (SDA-GR, µ) of each experimental culture was calculated as follows:
µ = [Ln (Ct2 / Ct1)] / (t2-t1)
where Ct1 and Ct2 are the abundance of the unialgal dinoflagellates at incubation times t1 and t2, respectively.
Here, the time for (t2-t1) is set to be 6d for the calculation of SDA-GR. During the 34d cultivation experiment, the maximum biomass yield (Ymax) of each bottle was determined as the unialgal concentration at the highest peak or maximum point on the population growth curve.
Table 1.
The test gradients of temperature (T, °C), salinity (S), and irradiance (L, µmol photons m-2 s-1) for the unialgal cultures of Ostreopsis sp. and Coolia canariensis native to Jeju coastal waters
| Gradient level | T (°C) | S | L (µmol photons m-2 s-1) |
| 1 | 15 | 15 | 10 |
| 2 | 20 | 20 | 20 |
| 3 | 25 | 25 | 50 |
| 4 | 30 | 30 | 100 |
| 5 | 35 | 35 | 200 |
| 6 | 300 |
Cytotoxicity of the Ostreopsis and Coolia cultures against Artemia Nauplii
For the cytotoxicity experiments, 10 Artemia nauplii were added to each test glass tube containing a 5-ml volume of cell-free filtrate or live cells of an EPD species in f/2 medium. Then, the cytotoxicity of the two EPD species against Artemia nauplii was determined by counting the number of Artemia nauplii killed daily in each glass tube for a 72h period and calculating the survival rate of the Artemia nauplii. Initial concentration of EPDs in each set of triplicate glass tube was adjusted to 0, 1000, and 5000 and 0, 5000, and 12000 cells ml-1 for Ostreopsis sp. and C. canariensis, respectively. The cell-free filtrate preparation in each glass tube also was also derived from the same initial EPD concentrations as above.
Spatiotemporal distribution of Ostreopsis and Coolia species in Jeju Coastal Waters
The vertical distribution of Ostreopsis and Coolia species was investigated at a sampling station off Moon-seom (33°13′39.32" N and 126°33′50.97" E), an islet near Seogwipo-si, Jeju Island (Fig. 1). Macroalgal substrates of the thalloid and crustose red alga Amphiroa sp. were collected from five different depths (0.5, 5, 10, 15, and 20 m) in June and October 2011.

Fig. 1.
An oceanographic map showing Korean seas surrounding Jeju Island (modified from the map published in 2011 by the Korea Hydrographic and Oceanographic Agency, Ministry of Oceans and Fisheries, Korea). The map in a separate box shows the sampling stations around Jeju Island and the Moom-seom, an islet in front of the arrowhead (scale bar = 10 miles)
The quantitative distribution of Ostreopsis and Coolia species in relation to habitat temperature and salinity was explored at six stations around Jeju Island (Fig. 1 and Table 2) based on the macroalgae sampling for even-numbered months of 2011. From each substrate macroalga of the whole 520 macroalgal samples the abundance of Ostreopsis and Coolia species was determined by microscopic observation and cell counting.
For the in situ sampling of each macroalgal substrate divers placed each macroalga in a plastic bottle, then fixed it with buffered 3% formaldehyde, and later shook the fixed sample vigorously to detach the dinoflagellate cells before final filtration through a 500 μm Nitex mesh. The filtrate containing dinoflagellates was stained with Calcofluor-white (Sigma Aldrich), and cells were observed and counted using a light microscope (Zeiss AxioCam HRc5, Carl Zeiss Ltd. Göttingen, Germany) and a Sedgwick- Rafter counting chamber. EPDs belonging to Coolia and Ostreopsis genus were counted to obtain abundance data per unit mass of the host macroalgae (cells (gram wet weight)-1, cells GWW-1).
Table 2.
Location and name of the 6 sampling stations for bimonthly sampling around Jeju Island. Total number of macroalgal (MA) samples in each station during a whole year is shown in the last column
3. Results and Discussion
Growth response to water temperature, salinity, and illuminance gradients by unialgal cultures of Ostreopsis sp. and Coolia canariensis
Ostreopsis sp. exhibited positive triplicate mean SDA-GR (6d averaged daily specific growth rate, µ) when the water temperature was lower than 35°C and peaked at 20°C. The growth rate of C. canariensis gradually increased as water temperature increased from 15°C to 25°C, but showed negative values at 30 and 35°C (Fig. 2). Trend of the triplicate mean Ymax (maximum biomass yield) along the temperature axis was similar to that of triplicate mean SDA-GR for both strains (Fig. 3). However, the preferred temperature (20°C in Figs. 2a and 3a) of Ostreopsis sp. with wider temperature tolerance was lower than that of the relatively more stenothermal C. canariensis with best performance at 25°C (Figs. 2a and 3a).

Fig. 2.
Mean maxiumum growth rates (mean GRmax) of the epiphytic dinoflagellate strains Ostreopsis sp. and C. canariensis (a–c) along the gradients of water temperatue (a), salinity (b) and light intensity (c). The mean GRmax is the mean of maximum SDA-GR (6d averaged daily specific growth rate) in each of the triplicate bottles during a 34d cultivation experiment. Error bar: standard error. Significant differences resulted from T-tests are indicated by asterisks (*: > 0.95, **: > 0.99) above the paired bars representing each environmental condition

Fig. 3.
Mean maxiumum biomass yields (mean Ymax) of the epiphytic dinoflagellate strains Ostreopsis sp. and C. canariensis (a–c) along the gradients of water temperatue (a), salinity (b) and light intensity (c). The mean Ymax is the mean of the Ymax in each of the triplicate bottles during a 34d cultivation experiment. Error bar: standard error. The sloid lines and dotted lines reflect the trend of Ymax variations along the gradients of water temperatue (a), salinity (b) and light intensity (c) represented by Ostreopsis sp. and C. canariensis, respectively
Negative growth and negligible biomass yield were found for both strains at low salinities (15 and 20), with the highest mean SDA-GR and Ymax met at salinities 35 and 30 in Ostreopsis sp. and C. canariensis, respectively (Figs. 2 and 3). At 15, the lowest salinity tested, the C. canariensis population exhibited very sharp decline compared to Ostreopsis sp. (Fig. 2b). Thus, C. canariensis seems to be more stenohaline than the Ostreopsis sp. strain (Figs. 2b and 3b).
C. canariensis showed positive growth at all 6 light intensities with the maximum of the triplicate mean SDA-GR at 50 µmol photons m-2 s-1 whereas negative growth of Ostreopsis sp. appeared only at 10 and 20 µmol photons m-2 s-1 (Fig. 2c). The Ymax of Ostreopsis sp. gradually increased at irradiances from 50 to 300 µmol photons m-2 s-1 (Fig. 3c). Although both the mean SDA-GR and Ymax of C. canariensis were positive at the 6 light intensities, Ymax at 200 and 300 µmol photons m-2 s-1 were markedly lower than at the other irradiances (Fig. 3c). Thus, Ostreopsis sp. appears to be better adapted to high light intensities than C. canariensis, which has a wide light-adapting range.
In situ spatiotemporal distribution of Ostreopsis and Coolia species in Jeju coastal waters
In the plane of temperature and salinity axes, the two balloon diagrams of Ostreopsis and Coolia abundances (Fig. 4a and 4b) among the 520 macroalgae samples collected at 6 stations during 2011 were quite distinct from each other (Fig. 4). In contrast to the large T-S (13.5–22.7°C, 29.2–34.1) space occupied by high Ostreopsis abundances, Coolia abundances were concentrated in the small T-S (16.2–22.6°C, 33.2–34.1) space (Fig. 4a and 4b). The T-S cores with the highest EPD abundance in Ostreopsis (20.3°C, 32.0) and Coolia (22.6°C, 34.1) differed significantly from each other. The above in situ distribution of Ostreopsis and Coolia matches strikingly well with the results from the experimental cultivation of the strains Ostreopsis sp. and C. canariensis, where maximum growth rate (GRmax) and Ymax were found at 20°C and 25°C in the strain Ostreopsis sp. and C. canariensis, respectively (Fig. 5a). Again, Ostreopsis species living in Jeju coastal waters was shown to be more eurythermal and with lower optimal temperatures than Coolia species (Fig. 4a and 4b). In addition, the gowth limit temperature (see the positive growth of Ostreopsis sp. strain at 30°C in Fig. 5a) supports the eurythermal character of Ostreopsis species inhabiting Jeju coastal waters.

Fig. 4.
Balloon diagrams of the epiphytic dinoflagellate (EPD) abundance from each of the 520 macroalgal substrates collected bi-monthly at 6 stations of Jeju coastal waters when plotted in the in the plane of water temperature axis and salinity axis. (a) Ostreopsis species. The dotted line indicates the T-S rectangular area with > 2500 Ostreopsis cells GWW-1. (b) Coolia species. The dotted line indicates the T-S rectangular area with > 1000 Coolia cells GWW-1
The highest mean SDA-GR of the experimental strains was found at salinity ranges of 30–35 in both Ostreopsis sp. and C. canariensis strains (Fig. 5b), which was also reflected by the in situ salinity for the distribution of high abundance core of Ostreopsis species (32.0) and Coolia species (34.1) (Fig. 4). Thus, Ostreopsis sp. strain with the highest GRmax at salinity 35 seems to be more euryhaline than C. canariensis strain (Coolia species with the highest GRmax at salinity 30 (Fig. 5b). The local adaptation of Ostreopsis species to lower salinities as well as the tolerance of in situCoolia species to narrower salinity ranges (Fig. 4) again reflect the euryhaline character of Ostreopsis species inhabiting coastal waters of Jeju.
Vertical distribution of Ostreopsis and Coolia species in Moon-seom, an islet of Jeju Island
EPD abundances on the thalloid and crustose red alga Amphiroa sp. at 4–5 water depths of the sampling station off Moon-seom showed vertically significant profiles (Fig. 6). In June, the abundance of Ostreopsis spp. rapidly decreased from a surface maximum of 226 to 3.5 cells GWW-1 at 15 m depth while Coolia spp. showed somewhat similar abundances from 5m to 15 m depth (Fig. 6a). The maximum abundance of Ostreopsis and Coolia in October was at 10 m depth, with extreme vertical variation in Ostreopsis compared to less pronounced vertical variation in Coolia (Fig. 6b). Abundance sums of all five genera including three other additional genera, Amphidinium, Gambierdiscus, and Prorocentrum, exhibited relatively rather even vertical profiles in both June and October (Fig. 6a and 6a). In both June and October, minimal Ostreopsis and Coolia were found at the deepest sampling depths (lowest light intensities) and surface (highest light intensities), respectively (Fig. 6). These results for the in situ EPD distribution appear to clearly reflect the growth response of clonal cultures to different light intensities in the experimental cultures with Ostreopsis sp. and C. canariensis strains (Figs. 2 and 3). Better adaption to high light intensity by Ostreopsis sp. strain and the contrasting adaptation to wider range of light intensities by C. canariensis strains in the experimental cultivation (Fig. 7) allows for a better understanding of the differences in the vertical profiles of in situ abundance between Ostreopsis and Coolia species in the Moon-seom (Fig. 6).

Fig. 6.
Vertical distribution of Ostreopsis and Coolia abundances (cells GWW-1) on the blades of a substrate red alga Amphiroa sp., respectively, in June (a) and October (b). The sum of the abundance of all the five genera (‘5 genera’ in a and b) exhibited vertically least variable profiles in both June and October. Error bar: standard error
Cytotoxicity of the Ostreopsis sp. and Coolia canariensis strains against Artemia Nauplii
Cytotoxicity against Artemia nauplii was undetectable at 12h for all experimental preparations and undetectable at 24h for low density preparations (5000 cells ml-1) of C. canariensis (Fig. 8). Cell filtrates were found to be less toxic than live cells for all the preparations except for one case (Fig. 8a), a low-density preparation of C. canariensis (5000 cells ml-1, at 36h). For the most toxic preparation (Ostreopsis sp., 5000 cells ml-1) (Fig. 8d), the median lethal time, LT50, was calculated to be 30.9 and > 57.0 h for live cell and cell filtrate treatments, respectively. On d2 and d3, the level of cytotoxicity against Artemia nauplii exerted by low-density (1000 cells ml-1) preparation (Fig. 8b) of Ostreopsis sp. was similar to that by the high-density (12000 cells ml-1) preparations (Fig. 8c) of C. canariensis (see treatments ‘Cc-12000’ and ‘Osp-1000’ in Fig. 9).
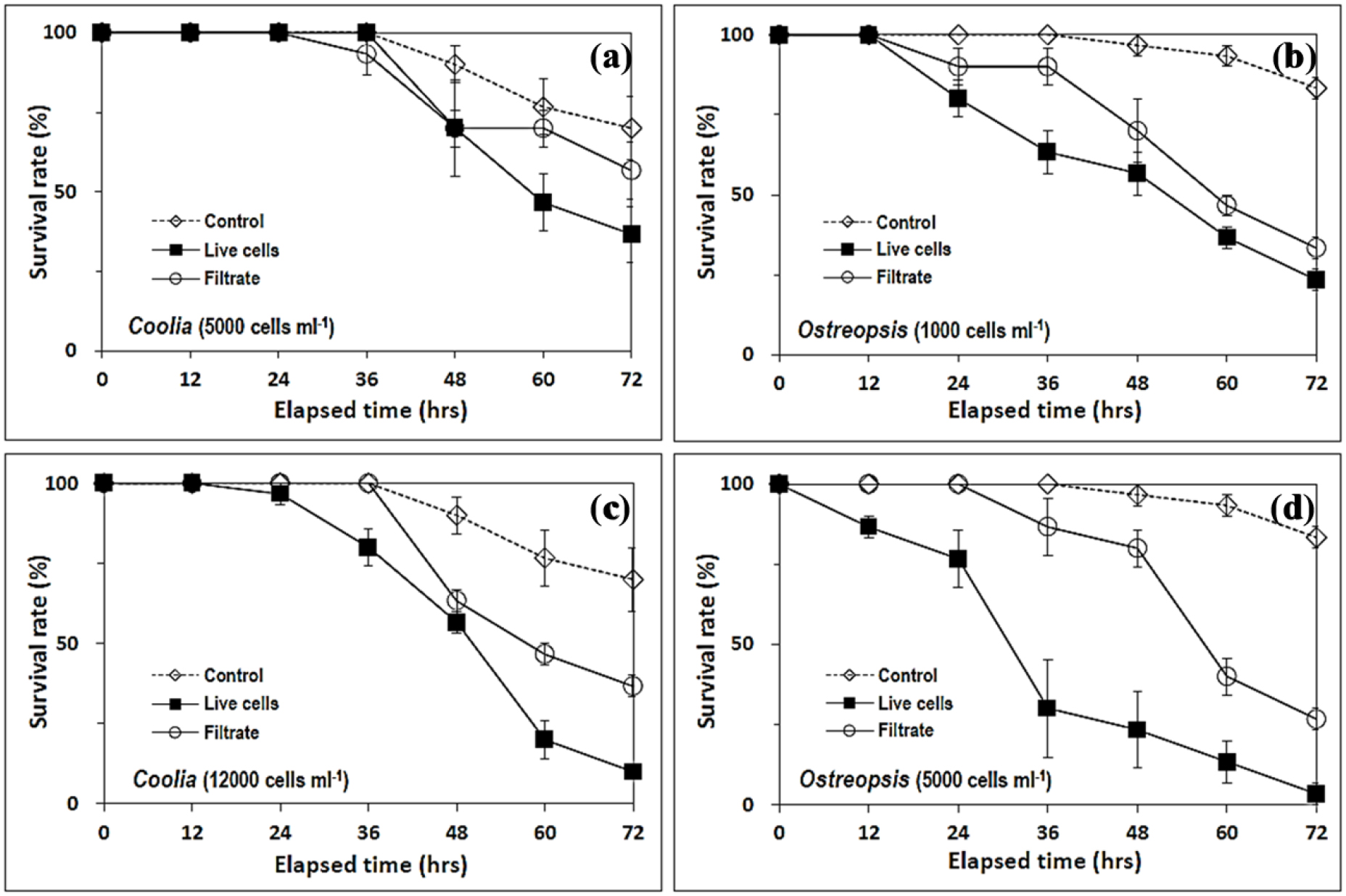
Fig. 8.
Survival rate (%) of Artemia nauplii in the live cell and cell-free filtrate treatments of Ostreopsis sp. and C. canariensis cultures. Over 72 h exposure, live cell and cell-free filtrate treatments with 5000 (a) and 12000 (c) cells ml-1 culture of C. canariensis strain and 1000 (b) and 5000 (d) cells ml-1 culture of Ostreopsis sp. strain were tested for Artemia nauplii cytotoxicity. Error bar: standard error

Fig. 9.
Triplicate mean lethality (%) at d1 (24 h), d2 (48 h), and d3 (72 h) of live cell and cell-free filtrate treatments of Ostreopsis sp. (Osp) and C. canariensis (Cc) cultures to Artemia larvae. Live cell (black bar) and cell-free filtrate treatments (empty bar) with 5000 (Cc-5000) and 12000 (Cc-12000) cells ml-1 culture of C. canariensis strain and 1000 (Osp-1000) and 5000 (Osp-5000) cells ml-1 culture of Ostreopsis sp. strain were tested for Artemia nauplii cytotoxicity. The lethality (%) in the bottles was adjusted to calibrate the lethality in control bottles after 36 h (Fig. 8) to be “0”
The high cytotoxicity of C. canariensis against Artemia nauplii was previously unknown. The cytotoxicity of C. canariensis against Artemia nauplii in this study was somewhat higher than that of C. canariensis isolated from Hong Kong waters (see Fig. 5 in Leung et al. 2017). Lysate solutions of C. canariensis at 0.5 mg ml-1 equivalent to 0.47 and 0.32 million cells ml-1 of the two Hong Kong strains, W039 and Ve011, respectively, was shown to be non-lethal against Artemia nauplii (Leung et al. 2017). However, for the prism larvae of sea urchin (Heliocidaris crassispina) the two Hong Kong strains of C. canariensis, W039 and Ve011, exhibited LC50 at lysate concentrations 0.082 and 0.064 mg ml-1 (equivalent to 78 and 40 thousand cells ml-1), respectively (Leung et al. 2017). Although the yessotoxin analogues, C56H78O18S2 and C58H86O18S2, were determined in the Guam strain G6 of C. canariensis, no toxic effects of the water-soluble fraction of strain G6 on Artemia was observed (Phua et al. 2021).
4. Conclusion
The cytotoxicity against Artemia nauplii (Fig. 8) as well as the growth responses to different water temperature, salinity, and light intensity (Figs. 2 and 3) were explored and compared through culture experiments of Ostreopsis sp. and C. canariensis strains from Jeju coastal waters. The difference in ecological characteristics of the two strains derived from the cultivation experiments was found to be in good agreement with the in situ distribution pattern of the corresponding EPD genera in Jeju coastal waters (Figs. 4 and 6).
Ostreopsis sp. strain with a lower preferred temperature (20°C) was also more temperature tolerant than the relatively stenothermal C. canariensis strain that performed best at 25°C (Figs. 2 and 5). The distribution pattern of Ostreopsis spp. and Coolia spp. in the 520 macroalgal samples collected bimonthly from 6 stations in Jeju coastal waters (Fig. 4) matched well with the eurythermal characteristics of Ostreopsis sp. strain in compared to C. canariensis strain (Figs. 2 and 5). The more stenohaline C. canariensis strain compared to the Ostreopsis sp. strain (Figs. 2 and 5) also matched well with a much narrower salinity range in the in situ distribution of Coolia spp. than that of Ostreopsis spp. in the 520 macroalgal samples (Fig. 4).
The preference of Ostreopsis sp. strain for high light intensities contrasted markedly with the better growth of C. canariensis strain at lower light intensities, including 10 and 20 µmol photons m-2 s-1, even though the Ymax of C. canariensis was significantly lower in high light intensities above 200 µmol photons m-2 s-1 (Figs. 2 and 3). Differences in light adaptation between the two strains (Figs. 2 and 3) are likely reflected in the in situ vertical profiles of Ostreopsis spp. and Coolia spp. from the thalloid and crustose red alga Amphiroa sp. off Moom-seom in June and October (Fig. 6). In October Coolia spp. exhibited a somewhat more even vertical distribution compared to the absence (‘0’) of Ostreopsis spp. at a depth of 20 m (Fig. 6b). Again, abundance of Ostreopsis spp. gradually decreased with increasing depth while the abundances of Coolia spp. peaked at 10 m in June (Fig. 6a).
Cytotoxicity against Artemia nauplii was detected as percent lethality for all preparations containing live cell as well as cell-free extracts of Ostreopsis sp. and C. canariensis strains during a 72 h incubation experiments. Lethality (%) to Artemia nauplii in the Ostreopsis sp. preparations at 1000 cells ml-1 (Osp-1000 in Fig. 9) was similar to C. canariensis preparations at 12000 cells ml-1 (Cc-12000 in Fig. 9) on d2 and d3. Thus, the cytotoxicity of C. canariensis against Artemia nauplii in this study was somewhat higher than that of C. canariensis isolated from Hong Kong waters (Leung et al. 2017). Such a high cytotoxicity of C. canariensis against Artemia larvae was previously not known. Therefore, both strains from Jeju coastal waters were found to be toxic to Artemia nauplii in this study, which is noteworthy in terms of food safety and leisure activities in Jeju coastal areas.
In conclusion, the growth characteristics of the two EPD strains under different temperature, salinity, and light intensity derived from the laboratory experiments were found to be very important in understanding the spatiotemporal distribution of EPD species and abundance in the Jeju subtidal habitats. In other words, the difference in growth characteristic of the two EPD strains was useful in explaining the contrasting spatial and temporal distribution patterns of Ostreopsis spp. and Coolia spp. in Jeju coastal waters. The strain C. canariensis isolated from Jeju coastal waters showed notably higher cytotoxicity against Artemia nauplii than previous reported. Therefore, in the near future, a new potential cytotoxicity risk may be introduced by C. canariensis inhabiting Jeju coastal waters, in addition to the present risks of the genus Ostreopsis, a currently well-known potentially toxic EPD species. In order to prepare for the hazards of EPD cytotoxicity in the coastal waters of Jeju Island in the future, it is necessary to further explore the contrasting ecological niches occupied by EPD species in relation to the cytotoxicity risks of the dominant EPD species.




